IS THE INDUSTRY PREPARED FOR ANOTHER YEAR OF RAPID GROWTH?
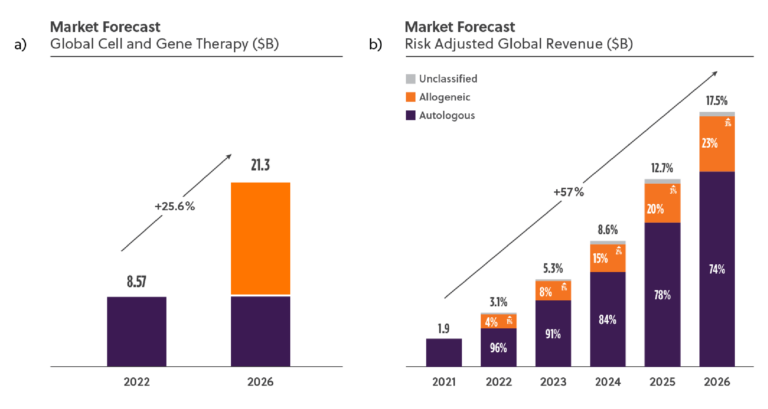
The cell and gene therapy (CGT) industry is expected to grow rapidly for the next 10 years with an increasing proportion of these treatments focused on allogeneic approaches according to industry analysts (Figure 1)1. Several factors are contributing to CGT’s rapid growth, including advances in research and development, a growing number of FDA approvals, and an increasing patient population expected to grow at approximately 20-30% year over year 2.
A sharp increase in the CGT industry’s demand for apheresis collection capacity has placed an incremental strain on the global supply chain. An estimated threefold increase in the apheresis collection is predicted in the United States alone by 2025 which outpaces current capacity. As one would expect, the supply gap introduces manufacturing bottlenecks which may lead to stalled progress, reduced access to CGTs, and longer wait times for patients3. Apheresis procedures are complex, time-consuming, and constrained by their own set of challenges – issues that add greater complexity to sourcing critical raw materials. Primary cell providers who rely on outsourced collections for their supply encounter overwhelming obstacles to address the growing demand. Industry-leading CGT developers gain key advantages by partnering with an apheresis provider that owns all aspects of their business, is vertically integrated, and has a proven track record of delivering on time – every time.
HOW WILL THE INDUSTRY GRAPPLE WITH SUPPLY CHAIN OBSTACLES?
Rapidly evolving market dynamics, coupled with the complexities of CGT treatments, implore biomedical researchers to address potential gaps in their supply chain network to mitigate future risks. For example, some CGT developers rely on hospitals and independent apheresis centres to supply the starting cellular material, while other developers rely on disjointed primary cell suppliers that outsource their collections to third-party blood banks. Many of these third-party facilities and outsourced suppliers are still learning/adapting to their evolving role as a key part of the overall CGT supply chain ecosystem. Varying levels of quality and consistency persist and could impede the industry’s growth if not properly identified by CGT developers. Challenges associated with CGT workflows include adequate quality management systems, standardisation of audits and training, variability of apheresis collections across sites, and transportation logistics – all of these aspects must be carefully managed 2.
THE TRUSTED APHERESIS SUPPLIER FOR 26+ YEARS
AllCells, a Discovery Life Sciences company, has dedicated substantial resources to proactively expand its apheresis infrastructure and donor-centric recruitment to keep pace with global industry growth. Since 1998, AllCells has established direct partnerships with CGT developers by offering highly characterised starting materials from its vertically integrated business model. All aspects of AllCells’ operations are under one roof. From collection, and processing, to shipment, CGT developers have benefited from this single source.
AllCells’ apheresis network is purposefully built for the CGT sector and is complemented by its best-in-class donor management services and analytical services. As the industry-leading partner for leukopaks and bone marrow tissue, its robust and harmonised quality management system has built-in risk mitigation measures to establish a secure supply chain, quality products, and consistency at scale. The CGT industry trusts AllCells to provide high quality and a smooth transition from R&D (research) to clinic applications (GMP), supporting long-term solutions throughout the CGT continuum. In 2022, AllCells ‘ donor collection network became the world’s largest apheresis provider following its acquisition by Discovery Life Sciences (Discovery). While still maintaining its central focus on providing end-to-end reliability and consistency, the AllCells of today is poised to ensure quality at scale for tomorrow – and beyond.
THE WORLD’S LARGEST APHERESIS CAPACITY WITH A 99% DELIVERY RATE
Donor-to-donor variability influences the final CGT product; therefore, it’s important to control the process to mitigate this inherent variability. Each collection facility in AllCells’ network is operationally integrated, staffed with experienced clinicians, and focused on maintaining a high standard of quality. Every facility also adheres to stringent quality management standards and best practices for downstream consistency.
AllCells is a recognised global service and quality leader due to its 99% deliverability rate and adherence to rigorous quality standards. With over 3,700 CGT-focused clinical trials underway globally, AllCells has invested significant resources to expand collection sites and increase its apheresis capacity to meet phase-appropriate requirements4. As a result, customers have access to tens of thousands of well-characterised, highly engaged, and recallable donors, coupled with in-house access to comprehensive analytical characterisation, including cell biology, genomic, and proteomic capabilities.
THE LARGEST APHERESIS CAPACITY COMPLEMENTED BY CHARACTERISATION FOR CELL AND GENE THERAPIES
At AllCells, sustaining a large and diverse network requires proactive engagement of potential donors by skilled and knowledgeable staff with expertise in donor selection. Critical factors are considered during donor screening and selection, such as age, weight, gender, ethnicity, human leukocyte antigen (HLA) type, medical history, and client-dependent criteria. An understanding of how these factors influence attributes like cell purity, yield, and transduction efficiency downstream is also crucial.
In addition to its facilities expansion, AllCells has scaled its donor digital marketing, specialised recruiting, and event-hosting efforts as well as increased its global donor operation workforce. In fact, 20% of AllCells’ employees — with over two decades of experience — are dedicated to donor operations specialising in donor recruitment and retention for the purpose of CGT development and commercialisation. And, with Discovery’s integrated analytical characterisation and multi-omic capabilities, AllCells donor pool is extensively characterised and annotated beyond just HLA typing. Understanding the correlation between the starting material attributes and the eventual quality of the CGT can uncover which criteria are critical to control5. Not only will access to this data enable smarter donor selection for faster timelines at scale, but it can also help better position CGT developers downstream to improve consistency in manufacturing processes that will ultimately lead to a more consistent final CGT product.
As an organisation, AllCells and Discovery will continue to expand their solutions alongside the CGT industry to help researchers overcome roadblocks. Combined with Discovery’s isolated immune cells, diseased human biospecimens, and multi-omic biomarker service capabilities, researchers and manufacturers have an end-to-end, integrated solution to streamline the advancement of new therapies from early research through to commercial manufacturing.
References
- Research and Markets. Global Cell and Gene Therapy Market Report. Accessed February 15, 2023. https://www.researchandmarkets.com/reports/5733866/
- Srivastava S and Mrithinjayam V. CGT Apheresis Service Market: Future Challenges. Cell & Gene. Published April 6, 2022. Accessed February 15, 2023. https://www.cellandgene.com/doc/cgt-apheresis-service-market-future-challenges-0001
- Association for the Advancement of Blood & Biotherapies. Managing Apheresis Unit Demand. Published October 13, 2022. Accessed February 15, 2023. https://www.aabb.org/news-resources/news/article/2022/10/13/aabb-news-managing-apheresis-unit-demand#
- American Society of Gene & Cell Therapy. 2022 Gene, Cell, & RNA Landscape Analysis Q4 Report. Published January 2023. Accessed February 15, 2023. https://asgct.org/global/documents/asgct_citeline-q4-2022-report_final.aspx
- Phacilitate. Ensuring a Robust Supply of Biomaterials to Meet a Growing Industry: An Interview with Dominic Clarke. Published Dec 30, 2021. Accessed February 15, 2023. https://www.phacilitate.com/ensuring-a-robust-supply-of-biomaterials-to-meet-a-growing-industry/
Originally posted by AllCells on https://allcells.com/the-worlds-largest-apheresis-network-serves-as-a-catalyst-to-advance-cell-and-gene-therapies/
Caltag Medsystems is the distributor of AllCells products in the UK and Ireland. If you have any questions about these products, please contact us.
