SB204990
Product Code:
AG-CR1-3697
AG-CR1-3697
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CR1-3697-M001 | 1 mg | £55.00 |
Quantity:
| AG-CR1-3697-M005 | 5 mg | £145.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
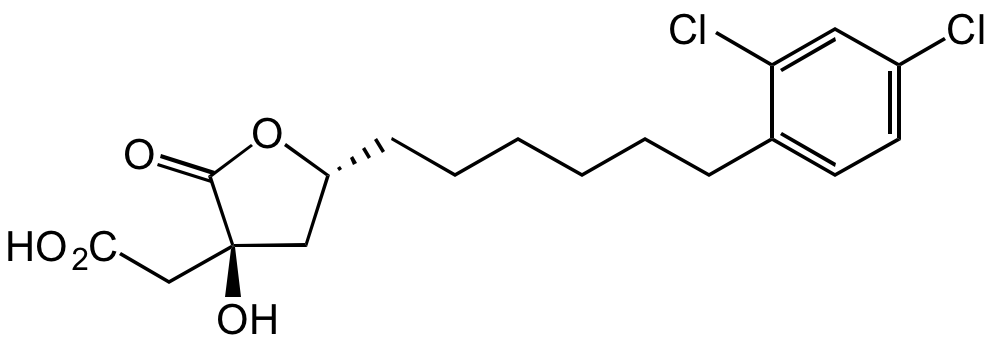
(3R,5S)-rel-5-[6-(2,4-Dichlorophenyl)hexyl]tetrahydro-3-hydroxy-2-oxo-3-furanacetic acid; SB-204990
Appearance:
White to off-white powder.
CAS:
154566-12-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C18H22Cl2O5/c19-13-8-7-12(15(20)9-13)5-3-1-2-4-6-14-10-18(24,11-16(21)22)17(23)25-14/h7-9,14,24H,1-6,10-11H2,(H,21,22)/t14-,18+/m1/s1
InChiKey:
YTRNLFYTHYWDAU-KDOFPFPSSA-N
Long Description:
Chemical. CAS: 154566-12-8. Formula: C18H22Cl2O5. MW: 389.3. Potent ATP citrate lyase (ACLY) inhibitor. ATP citrate lyase (ACLY), a key enzyme for lipid synthesis, is frequently overexpressed or activated in cancer to promote lipid synthesis and tumor progression. Cell permeable prodrug form of SB201076. Hypolipidemic agent. Fatty acid synthesis and cholesterol synthesis inhibitor in a dose-dependent manner. Useful agent for immunometabolism research. Orally active in vivo. Shown to block fatty acid synthesis and cancer cell growth in pre-clinical studies.
MDL:
MFCD28127018
Molecular Formula:
C18H22Cl2O5
Molecular Weight:
389.3
Package Type:
Vial
Product Description:
Potent ATP citrate lyase (ACLY) inhibitor. ATP citrate lyase (ACLY), a key enzyme for lipid synthesis, is frequently overexpressed or activated in cancer to promote lipid synthesis and tumor progression. Cell permeable prodrug form of SB201076. Hypolipidemic agent. Fatty acid synthesis and cholesterol synthesis inhibitor in a dose-dependent manner. Useful agent for immunometabolism research. Orally active in vivo. Shown to block fatty acid synthesis and cancer cell growth in pre-clinical studies.
Purity:
>95%
SMILES:
O=C1[C@](CC(O)=O)(O)C[C@@H](CCCCCCC2=CC=C(Cl)C=C2Cl)O1
Solubility Chemicals:
Soluble in DMSO, ethanol or DMF.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
ATP-Citrate lyase as a target for hypolipidemic intervention. 2. Synthesis and evaluation of (3R,5S)-omega-substituted-3-carboxy-3, 5-dihydroxyalkanoic acids and their gamma-lactone prodrugs as inhibitors of the enzyme in vitro and in vivo: A.D. Gribble, et al.; J. Med. Chem. 41, 3582 (1998) | The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076: N.J. Pearce, et al.; Biochem. J. 15, 334 (1998) | ATP citrate lyase inhibition can suppress tumor cell growth: G. Hatzivassiliou, et al.; Cancer Cell 8, 311 (2005) | Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression: C. Zhang, et al.; Genes Dev. 30, 1956 (2016)
Related Products
| Product Name | Product Code | Supplier | C75 (FASN Inhibitor) | AG-CR1-2904 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|