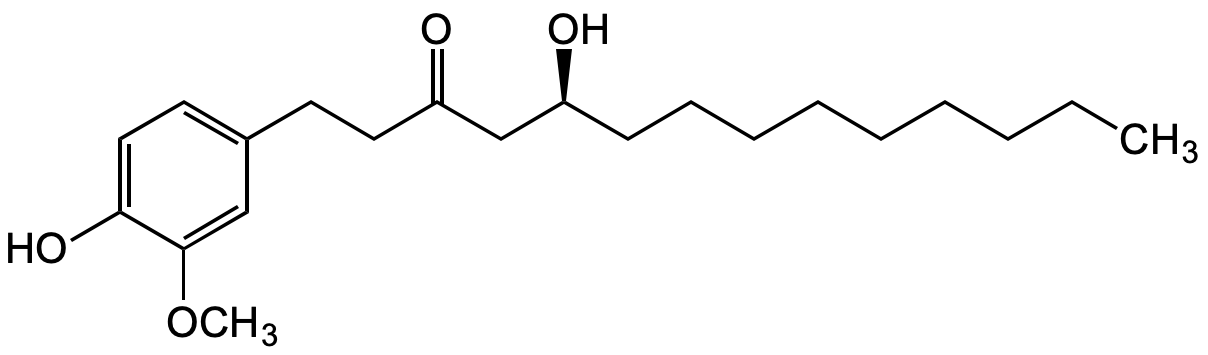
[10]-Gingerol
Product Code:
CDX-G0198
CDX-G0198
Host Type:
Plant
Plant
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
Short Term: +20°C, Long Term: +4°C
Short Term: +20°C, Long Term: +4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-G0198-M005 | 5 mg | £145.00 |
Quantity:
| CDX-G0198-M010 | 10 mg | £267.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
(S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-tetradecanone; 10-Gingerol; (S)-[10]-Gingerol
Appearance:
Light-yellow powder.
CAS:
23513-15-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C21H34O4/c1-3-4-5-6-7-8-9-10-18(22)16-19(23)13-11-17-12-14-20(24)21(15-17)25-2/h12,14-15,18,22,24H,3-11,13,16H2,1-2H3/t18-/m0/s1
InChiKey:
AIULWNKTYPZYAN-SFHVURJKSA-N
Long Description:
Chemical. CAS: 23513-15-7. Formula: C21H34O4. MW: 350.49. Isolated from plant source. 10-Gingerol is originally found in species of Zingiber. It exhibits antiemetic, anticancer, antioxidative, anti-inflammatory and antibiotic activities. 10-Gingerol increases radical scavenging of superoxide and hydroxyl radicals, inhibits oxidative burst activity and decreases expression of NO and PGE2 in vitro. It exhibits antioxidant behaviour towards lipids and is effective in suppressing obesity and adipose tissue inflammation. 10-Gingerol also inhibits 5-HT3 receptors. Additionally, 10-gingerol inhibits proliferation in different cancer cells. Shwon to suppresses IL-2-induced proliferation of T lymphocytes. This compound also displays antibacterial efficacy against gram negative bacteria such as Porphyromonas and Prevotella, antifungal and antiparasitic activity.
MDL:
MFCD01682694
Molecular Formula:
C21H34O4
Molecular Weight:
350.49
Package Type:
Vial
Product Description:
10-Gingerol is originally found in species of Zingiber. It exhibits antiemetic, anticancer, antioxidative, anti-inflammatory and antibiotic activities. 10-Gingerol increases radical scavenging of superoxide and hydroxyl radicals, inhibits oxidative burst activity and decreases expression of NO and PGE2 in vitro. It exhibits antioxidant behaviour towards lipids and is effective in suppressing obesity and adipose tissue inflammation. 10-Gingerol also inhibits 5-HT3 receptors. Additionally, 10-gingerol inhibits proliferation in different cancer cells. Shwon to suppresses IL-2-induced proliferation of T lymphocytes. This compound also displays antibacterial efficacy against gram negative bacteria such as Porphyromonas and Prevotella, antifungal and antiparasitic activity.
Purity:
>98% (HPLC)
SMILES:
OC1=CC=C(CCC(C[C@@H](O)CCCCCCCCC)=O)C=C1OC
Solubility Chemicals:
Soluble in DMSO (25mg/ml), DMF (30mg/ml), ethanol (15mg/ml) or methanol (5mg/ml).
Source / Host:
Isolated from plant source.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
Documents
References
(1) S. Chrubasik, et al.; Phytomedicine 12, 684 (2005) | (2) H. Abdel-Aziz, et al.; Eur. J. Pharmacol. 530, 136 (2006) | (3) M. Park, et al.; Phytother. Res. 22, 1446 (2008) | (4) C.Y. Chen, et al.; Molecules 14, 959 (2009) | (5) S. Dugasani, et al.; J. Ethnopharmacol. 127, 515 (2010) | (6) S.C. Ho, et al.; Food Chem. 141, 3183 (2013) | (7) M.A. Bakht, et al.; Asian Pac. J. Trop. Med. 4, 329 (2014) | (8) M. Bernard, et al.; Phytother. Res. 29, 1707 (2015) | (9) M.M. Bernard, et al.; Exp. Mol. Pathol. 102, 370 (2017) | (10) F. Zhang, et al.; Food Funct. 8, 2635 (2017) (Review) | (11) W. Si, et al.; Food Chem. 239, 1117 (2018) | (12) A.M. Fuzer, et al.; Anticancer Agents Med. Chem. 19, 645 (2019) | (13) Y.W. Fu, et al.; Vet. Parasitol. 265, 74 (2019)