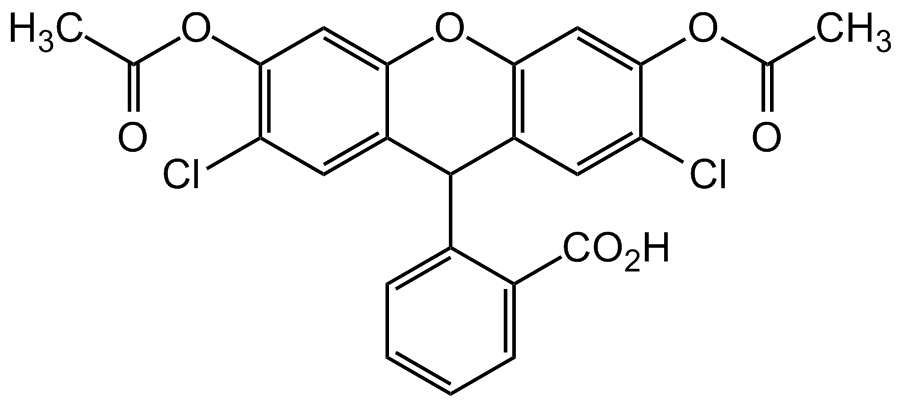
2',7'-Dichlorofluorescein diacetate
Product Code:
CDX-D0064
CDX-D0064
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Short Term: +4°C, Long Term: -20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-D0064-M050 | 50 mg | £41.00 |
Quantity:
| CDX-D0064-M250 | 250 mg | £108.00 |
Quantity:
| CDX-D0064-G001 | 1 g | £255.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
H2DCFDA; 2',7'-Dichlorodihydrofluorescein diacetate; Dichlorofluorescin DA; DCFDA; DCFH; DCFH-DA
Appearance:
White to off-white powder.
CAS:
4091-99-0
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C24H16Cl2O7/c1-11(27)31-21-9-19-15(7-17(21)25)23(13-5-3-4-6-14(13)24(29)30)16-8-18(26)22(32-12(2)28)10-20(16)33-19/h3-10,23H,1-2H3,(H,29,30)
InChiKey:
PXEZTIWVRVSYOK-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 4091-99-0. Formula: C24H16Cl2O7. MW: 487.29. Dichlorofluorescin diacetate (H2DCFDA) is a chemically reduced analog of fluorescein, used as an indicator for reactive oxygen species (ROS) in cells. The cell permeable non-fluorescent H2DCFDA is cleaved by cellular esterases and oxidation, and converted to the highly fluorescent 2',7'-dichlorofluorescein. H2DCFDA might be reactive toward a broad range of oxidizing reactions that may be increased during intracellular oxidant stress. Peroxynitrite is an efficient mediator of this oxidation and neither NO, superoxide, nor hydrogen peroxide alone appear to oxidize DCFH. This probe is widely used to monitoring cellular redox processes. Used as a component/substrate of the 2?,7?-dichlorofluorescein diacetate (DCFH-DA) assay to quantitate reactive oxygen species (ROS). Can detect reactive oxygen species in live cells. Spectral data: lambdaEx=504nm/lambdaEm=529nm.
MDL:
MFCD00128955
Molecular Formula:
C24H16Cl2O7
Molecular Weight:
487.29
Package Type:
Vial
Product Description:
Dichlorofluorescin diacetate (H2DCFDA) is a chemically reduced analog of fluorescein, used as an indicator for reactive oxygen species (ROS) in cells. The cell permeable non-fluorescent H2DCFDA is cleaved by cellular esterases and oxidation, and converted to the highly fluorescent 2',7'-dichlorofluorescein. H2DCFDA might be reactive toward a broad range of oxidizing reactions that may be increased during intracellular oxidant stress. Peroxynitrite is an efficient mediator of this oxidation and neither NO, superoxide, nor hydrogen peroxide alone appear to oxidize DCFH. This probe is widely used to monitoring cellular redox processes. Used as a component/substrate of the 2?,7?-dichlorofluorescein diacetate (DCFH-DA) assay to quantitate reactive oxygen species (ROS). Can detect reactive oxygen species in live cells. Spectral data: lambdaEx=504nm/lambdaEm=529nm.
Purity:
>95% (HPLC)
SMILES:
ClC1=C(OC(C)=O)C=C2C(C(C3=CC=CC=C3C(O)=O)C(C=C(Cl)C(OC(C)=O)=C4)=C4O2)=C1
Solubility Chemicals:
Soluble in DMSO (30mg/ml) or ethanol(5mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) A.S. Ferrer, et al.; Anal. Biochem. 187, 129 (1990) | (2) C.P. LeBel, et al.; Toxicol. Appl. Pharmacol. 104, 17 (1990) | (3) A.R. Rosenkranz, et al.; J. Immunol. Meth. 156, 39 (1992) | (4) F. Perez-Garcia, et al.; Life Sci. 59, 2033 (1996) | (5) J.P. Crow; Nitric Oxide 1, 145 (1997) | (6) N.W. Kooy, et al.; Free Rad. Res. 27, 245 (1997) | (7) C. Gabriel, et al.; J. Pharmacol. Toxicol. Meth. 38, 93 (1997) | (8) O. Myhre, et al.; Biochem. Pharmacol. 65, 1575 (2003) | (9) D. Figueroa, et al.; J. Pharmacol. Toxicol. Meth. 94, 26 (2018)