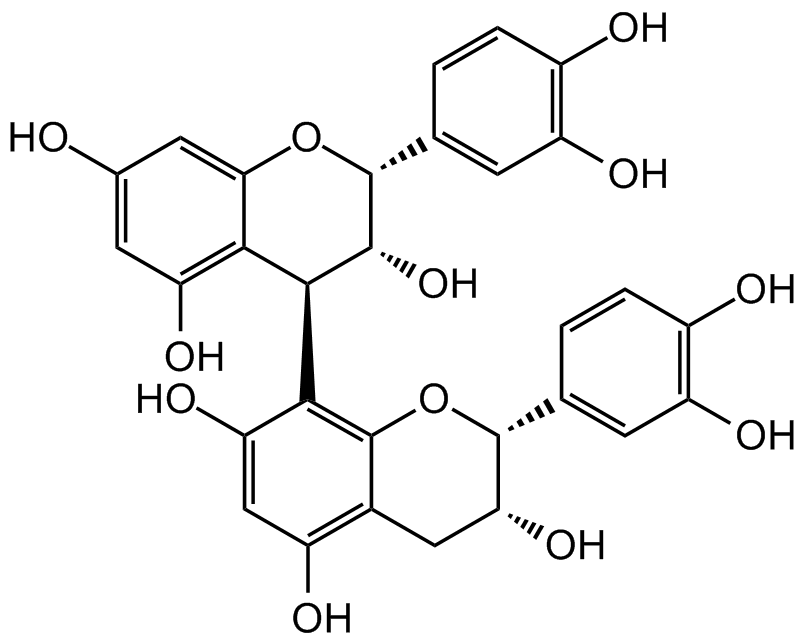
Procyanidin B2
Product Code:
CDX-P0591
CDX-P0591
Host Type:
Plant
Plant
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short term: +20°C, Long term: +4°C
Short term: +20°C, Long term: +4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-P0591-M001 | 1 mg | £68.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
(-)-Epicatechin (4beta-8)-(-)-epicatechin; 4,8'-Bi-[(+)-epicatechin]; cis,cis'-4,8'-Bi-(3,3',4',5,7-pentahydroxy-flavan); Proanthocyanidin B2; Procyanidol B2
Appearance:
Light beige/brown to beige/brown powder.
CAS:
29106-49-8
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C30H26O12/c31-13-7-20(37)24-23(8-13)41-29(12-2-4-16(33)19(36)6-12)27(40)26(24)25-21(38)10-17(34)14-9-22(39)28(42-30(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,22,26-29,31-40H,9H2/t22-,26-,27-,28-,29-/m1/s1
InChiKey:
XFZJEEAOWLFHDH-NFJBMHMQSA-N
Long Description:
Chemical. CAS: 29106-49-8. Formula: C30H26O12. MW: BD9837. Procyanidin B2, one of the most representative naturally occurring edible pigments, is usually isolated from the seeds, fruits and leaves of lots of common plants, such as grapes or apples. Procyanidin B2 has numerous bioactivities, including anti-inflammatory, anti-oxidative stress, neuroprotective, lipid metabolism regulating, wound healing, anti-diabetic, metabolic syndrome regulating, anti-atherosclerotic, anti-adipogenesis, anti-viral and anti-cancer properties. Procyanidin B2 targets and mediates several signaling pathways like NF-kappaB, MAPK, PI3K/Akt, NLRP3, apoptotic axis, VEGF/VEGFR2, PPARgamma and Nrf-2/HO-1. Procyanidin B2 possesses the potential to treat/prevent a wide range of human diseases, such as diabetes mellitus, diabetic complications, atherosclerosis, cancers and non-alcoholic fatty liver disease.
MDL:
MFCD01861513
Molecular Formula:
C30H26O12
Molecular Weight:
578.52
Package Type:
Vial
Product Description:
Procyanidin B2, one of the most representative naturally occurring edible pigments, is usually isolated from the seeds, fruits and leaves of lots of common plants, such as grapes or apples. Procyanidin B2 has numerous bioactivities, including anti-inflammatory, anti-oxidative stress, neuroprotective, lipid metabolism regulating, wound healing, anti-diabetic, metabolic syndrome regulating, anti-atherosclerotic, anti-adipogenesis, anti-viral and anti-cancer properties. Procyanidin B2 targets and mediates several signaling pathways like NF-kappaB, MAPK, PI3K/Akt, NLRP3, apoptotic axis, VEGF/VEGFR2, PPARgamma and Nrf-2/HO-1. Procyanidin B2 possesses the potential to treat/prevent a wide range of human diseases, such as diabetes mellitus, diabetic complications, atherosclerosis, cancers and non-alcoholic fatty liver disease.
Purity:
>90% (HPLC)
SMILES:
O[C@@H]1[C@@H](C2=C3C(C[C@@H](O)[C@@H](C4=CC(O)=C(O)C=C4)O3)=C(O)C=C2O)C5=C(O)C=C(O)C=C5O[C@@H]1C6=CC(O)=C(O)C=C6
Solubility Chemicals:
Soluble in DMSO, ethanol, DMF (all 30mg/ml) or water (10mg/ml).
Source / Host:
Isolated from plant.
Transportation:
Non-hazardous
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
Documents
References
(1) M.M. Avelar & C.M.C.P. Gouvea; Indian J. Pharm. Sci. 74, 351 (2012) | (2) H. Yang, et al.; Biochem. Pharmacol. 92, 599 (2014) | (3) T.C. Sutcliffe, et al.; Antioxidants 6, 77 (2017) | (4) S. Gopalakrishnan, et al.; IUBMB Life 70, 445 (2018) | (5) J. Feng, et al.; J. Cell. Mol. Med. 23, 6479 (2019) | (6) Y. Tian, et al.; Front. Immunol. 10, 1895 (2019) | (7) X. Zhu, et al.; Antioxid. Redox Signal. 35, 75 (2021) | (8) L. Liu, et al.; Pharmacol. Res. 177, 106127 (2022) | (9) M. Xu, et al.; Anim. Biotechnol. 33, 346 (2022) | (10) Q. Sun, et al.; Bioengineered 13, 6500 (2022) | (11) J. Liu, et al.; Nutrients 14, 1756 (2022) | (12) J. Zhao, et al.; Cell Death Dis. 13, 594 (2022) | (13) H. Wang, et al.; Int. J. Mol. Sci. 23, 7769 (2022) | (14) W. Cai, et al.; Int. Immunopharmacol. 113, 109336 (2022) | (15) Y.P. Zou, et al.; J. Orthop. Res. 41, 1555 (2023) | J. Chen, et al.; Food Chem. 420, 136101 (2023)