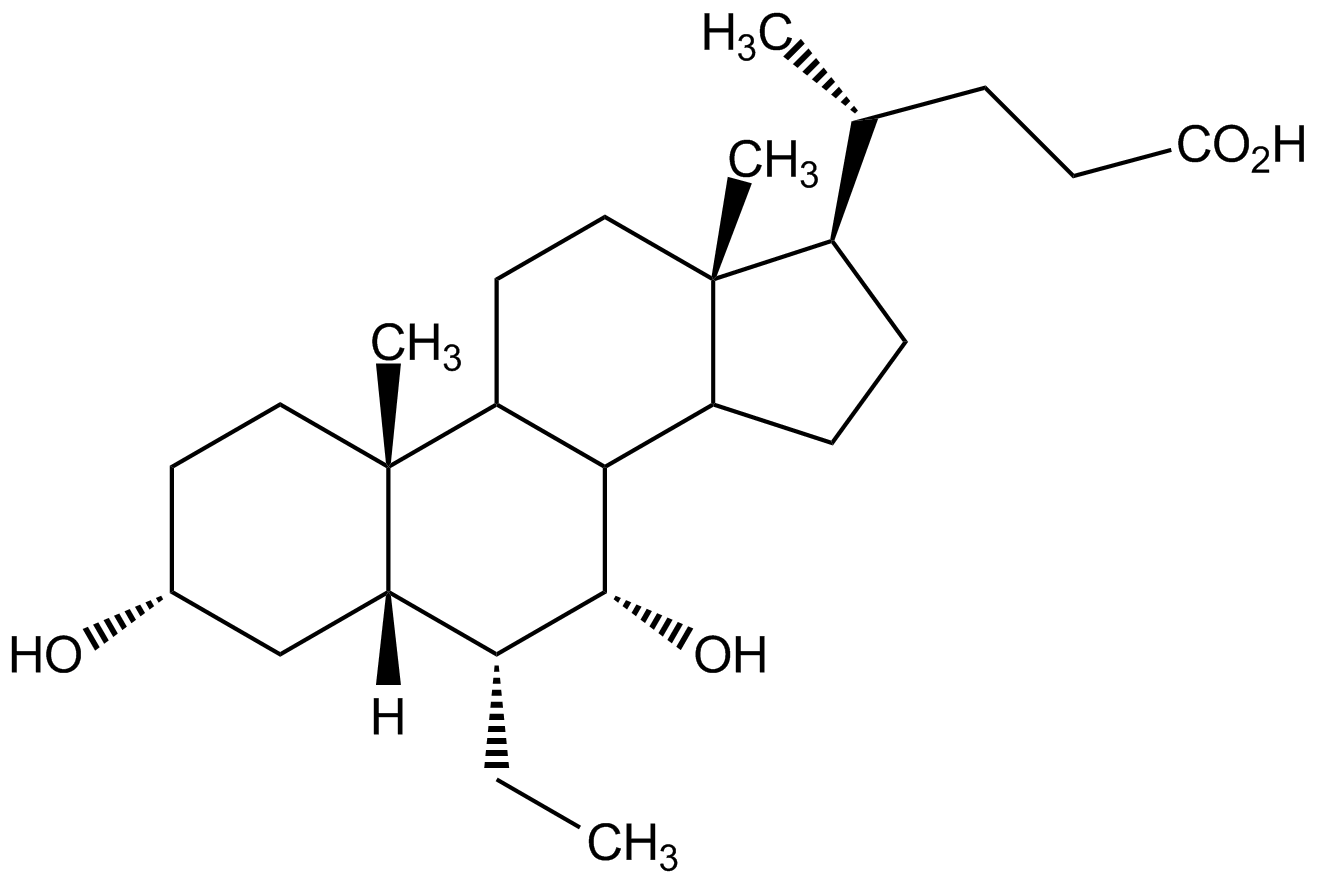
6-ECDCA
Product Code:
AG-CR1-3560
AG-CR1-3560
Regulatory Status:
RUO
RUO
Shipping:
-20°C
-20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CR1-3560-M005 | 5 mg | £60.00 |
Quantity:
| AG-CR1-3560-M025 | 25 mg | £130.00 |
Quantity:
| AG-CR1-3560-M100 | 100 mg | £290.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
6alpha-Ethyl-chenodeoxycholic acid; 6-alpha-ECDC; Obeticholic acid; INT-747; OCA
Appearance:
White solid.
CAS:
459789-99-2
EClass:
32160000
Form (Short):
liquid
InChi:
1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19?,20?,21+,23?,24-,25-,26-/m1/s1
InChiKey:
ZXERDUOLZKYMJM-AMGBFIHRSA-N
Long Description:
Chemical. CAS: 459789-99-2. Formula: C26H44O4. MW: 420.6. Potent and selective FXR agonist (EC50= 99nM). Induces SHP in HSCs to suppress TIMP-1 expression. Apoptosis inducer. Protects against liver fibrosis development in rat in vivo. Displays anticholeretic activity in rat in vivo. Promotes preadipocyte differentiation. Regulates adipogenesis and insulin signaling in vivo. Inhibits vascular smooth muscle cell inflammation and migration.
MDL:
MFCD16621104
Molecular Formula:
C26H44O4
Molecular Weight:
420.6
Package Type:
Vial
Product Description:
Potent and selective farnesoid X receptor FXR agonist (EC50= 99nM) [1]. Induces SHP in HSCs to suppress TIMP-1 expression [2]. Apoptosis inducer [2]. Protects against liver fibrosis development in rat in vivo [2]. Displays anticholeretic activity in rat in vivo [3]. Promotes preadipocyte differentiation [4]. Regulates adipogenesis and insulin signaling in vivo [4]. Inhibits vascular smooth muscle cell inflammation and migration [5].
Purity:
>95% (NMR)
SMILES:
[H][C@@]12C[C@H](O)CC[C@]1(C)C1CC[C@]3(C)[C@H](CCC3C1[C@H](O)[C@@H]2CC)[C@H](C)CCC(O)=O
Solubility Chemicals:
Soluble in ethanol, methanol, or DMSO. Slightly soluble in water, dichloromethane and acetone.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
6r-Ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity: R. Pellicciari, et al.; J. Med. Chem. 45, 3569 (2002) | A farnesoid X receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis: S. Fiorucci, et al.; J. Pharmacol. Exp. Ther. 314, 584 (2005) | Protective effects of 6-ethyl chenodeoxycholic acid, a farnesoid X receptor ligand, in estrogen-induced cholestasis: S. Fiorucci, et al.; J. Pharmacol. Exp. Ther. 313, 604 (2005) | The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo: G. Rizzo, et al.; Mol. Pharmacol. 70, 1164 (2006) | Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration: Y.T. Li, et al.; Arterioscler. Thromb. Vasc. Biol. 27, 2606 (2007) | Pharmacotoxicology of clinically-relevant concentrations of Obeticholic Acid in an organotypic human hepatocyte system: A. Dash, et al.; Toxicol. in vitro 39, 93 (2017) | Computational discovery and experimental verification of farnesoid X receptor agonist auraptene to protect against cholestatic liver injury: X. Gao, et al.; Biochem. Pharmacol. 146, 127 (2017) | Yangonin protects against cholestasis and hepatotoxity via activation of farnesoid X receptor in vivo and in vitro: X. Gao, et al.; Toxicol. Appl. Pharmacol. 348, 105 (2018)
Related Products
| Product Name | Product Code | Supplier | Stigmasterol | AG-CN2-0412 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|