No additional charges, what you see is what you pay! *
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available!
Contact us to find what you can save.
This product comes from:
Switzerland.
Typical lead time:
10-14 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
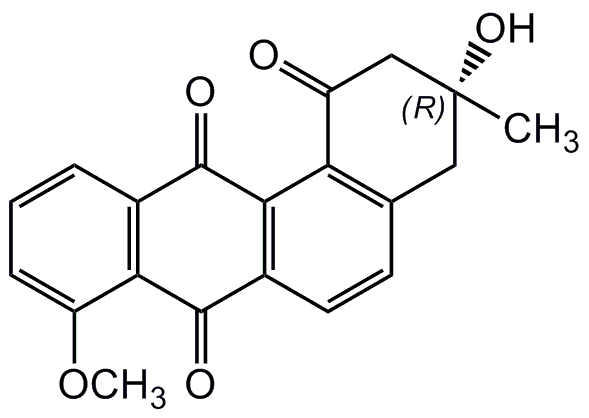
6-Desoxy-8-O-methylrabelomycin; (R)-3-Hydroxy-8-methoxy-3-methyl-3,4-dihydro-tetraphene-1,7,12(2H)-trione
Yellow solid.
117620-87-8
32160000
solid
GHS07
Protect from light when in solution.
H302, H312, H319
InChI=1S/C20H16O5/c1-20(24)8-10-6-7-12-17(15(10)13(21)9-20)19(23)11-4-3-5-14(25-2)16(11)18(12)22/h3-7,24H,8-9H2,1-2H3/t20-/m1/s1
XZLGWJORNHETKI-HXUWFJFHSA-N
Chemical. CAS: 117620-87-8. Formula: C20H16O5. MW: 336.3. Isolated from Streptomyces sp. Gö 40/14. Antibiotic. Angucyclinone. Antibacterial and antifungal.
MFCD01668121
C20H16O5
336.3
Plastic Vial
P270, P280, P301, P312, P302, P352, P312
Antibiotic. Angucyclinone. Antibacterial and antifungal.
>98% (HPLC)
Warning
COC1=CC=CC2=C1C(=O)C1=C(C2=O)C2=C(C[C@@](C)(O)CC2=O)C=C1
Soluble in DMSO, methanol or acetone.
Isolated from Streptomyces sp. G? 40/14.
Non-hazardous
Natural Products/Extracts
12352200
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.