Compound E
Product Code:
AG-CR1-0081
AG-CR1-0081
Regulatory Status:
RUO
RUO
Shipping:
-20°C
-20°C
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CR1-0081-C250 | 250 ug | £80.00 |
Quantity:
| AG-CR1-0081-M001 | 1 mg | £175.00 |
Quantity:
| AG-CR1-0081-M005 | 5 mg | £610.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
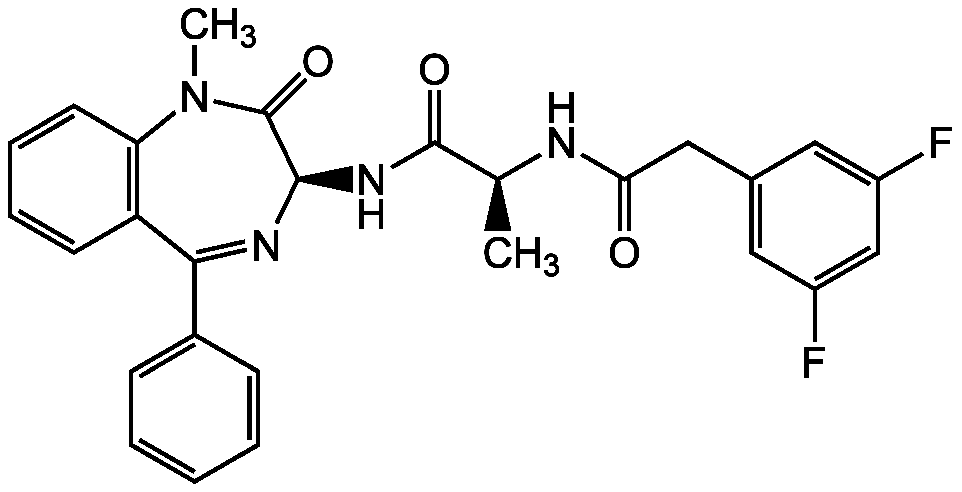
gamma-Secretase Inhibitor XXI; (2S)-2-([(3,5-Difluorophenyl)acetyl]amino)-N-[(3S)-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-3-yl]propanamide
Appearance:
White to off-white solid.
CAS:
209986-17-4
EClass:
32160000
Form (Short):
liquid
InChi:
InChI=1S/C27H24F2N4O3/c1-16(30-23(34)14-17-12-19(28)15-20(29)13-17)26(35)32-25-27(36)33(2)22-11-7-6-10-21(22)24(31-25)18-8-4-3-5-9-18/h3-13,15-16,25H,14H2,1-2H3,(H,30,34)(H,32,35)/t16-,25+/m0/s1
InChiKey:
JNGZXGGOCLZBFB-IVCQMTBJSA-N
Long Description:
Chemical. CAS: 209986-17-4. Formula: C27H24F2N4O3. MW: 490.5. Cell permeable, potent, selective, non-transition state and non-competitive gamma-secretase inhibitor. Notch processing inhibitor. Only weakly affects presenilinase activity.
MDL:
MFCD04974531
Molecular Formula:
C27H24F2N4O3
Molecular Weight:
490.5
Package Type:
Vial
Product Description:
Cell permeable, potent, selective, non-transition state and non-competitive gamma-secretase inhibitor. Notch processing inhibitor. Only weakly affects presenilinase activity.
Purity:
>98% (HPLC)
SMILES:
C[C@H](NC(=O)CC1=CC(F)=CC(F)=C1)C(=O)N[C@H]1N=C(C2=CC=CC=C2)C2=C(C=CC=C2)N(C)C1=O
Solubility Chemicals:
Soluble in DMSO.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
Presenilin-1 and -2 are molecular targets for gamma-secretase inhibitors: D. Seiffert, et al.; J. Biol. Chem. 275, 34086 (2000) | Presenilin-dependent gamma-secretase activity modulates thymocyte development: P. Doerfler, et al.; PNAS 98, 9312 (2001) | Pharmacological knock-down of the presenilin 1 heterodimer by a novel gamma -secretase inhibitor: implications for presenilin biology: D. Beher, et al.; J. Biol. Chem. 276, 45394 (2001) | gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase: C.Y. Ni, et al.; Science 294, 2179 (2001) | Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor: K.M. Jung, et al.; J. Biol. Chem. 278, 42161 (2003) | Identification of a new presenilin-dependent zeta-cleavage site within the transmembrane domain of amyloid precursor protein: G. Zhao, et al.; J. Biol. Chem. 279, 50647 (2004) | Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia: A.P. Weng, et al.; Science 306, 269 (2004) | Determination of guinea-pig cortical gamma-secretase activity ex vivo following the systemic administration of a gamma-secretase inhibitor: S. Grimwood, et al.; Neuropharmacology 48, 1002 (2005) | Jagged/Notch signalling is required for a subset of TGFbeta1 responses in human kidney epithelial cells: K.C. Nyhan, et al.; Biochim. Biophys. Acta 1803, 1386 (2010) | Investigating the amyloid-beta enhancing effect of cGMP in neuro2a cells: E. Calcagno, et al.; Mech. Ageing Dev. 166, 1 (2017)