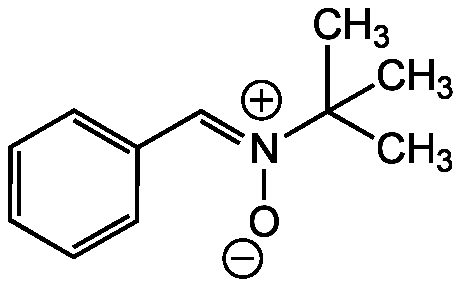
N-tert-Butyl-alpha-phenylnitrone [PBN]
Product Code:
CDX-B0269
CDX-B0269
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-B0269-G001 | 1 g | £65.00 |
Quantity:
| CDX-B0269-G005 | 5 g | £230.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Phenyl N-t-butylnitrone; N-tert-Butyl-alpha-phenylnitrone; N-Benzylidene-N-(2-methyl-2-propanyl)amine oxide; (Z)-N-Benzylidene-2-methylpropan-2-amine oxide
Appearance:
Off-white to white crystals.
CAS:
3376-24-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C11H15NO/c1-11(2,3)12(13)9-10-7-5-4-6-8-10/h4-9H,1-3H3
InChiKey:
IYSYLWYGCWTJSG-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 3376-24-7. Formula: C11H15NO. MW: 177.2. Synthetic. Cell permeable spin trap commonly used in free radical research for both in vivo and in vitro studies. It protects against oxidative damage caused by various inflammatory events, demonstrating neuroprotective, anti-anging, and antidiabetic effects. PBN has been shown to inhibit LPS-induced NF-kappaB DNA binding activity, inhibit COX-2 catalytic activity, inhibit lipid peroxidation in rat liver microsomes and prevent the induction of inducible nitric oxide synthase (iNOS). Shows anti-cancer activity in several experimental cancer models.
MDL:
MFCD00008799
Molecular Formula:
C11H15NO
Molecular Weight:
177.2
Package Type:
Vial
Product Description:
Cell permeable spin trap commonly used in free radical research for both in vivo and in vitro studies. It protects against oxidative damage caused by various inflammatory events, demonstrating neuroprotective, anti-anging, and antidiabetic effects. PBN has been shown to inhibit LPS-induced NF-kappaB DNA binding activity, inhibit COX-2 catalytic activity, inhibit lipid peroxidation in rat liver microsomes and prevent the induction of inducible nitric oxide synthase (iNOS). Shows anti-cancer activity in several experimental cancer models.
Purity:
>98% (GC)
SMILES:
CC(C)(C)[N+]([O-])=CC1=CC=CC=C1
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Spin Labels
UNSPSC Number:
12352100
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) R.A. Floyd; FASEB J. 4, 2587-4597 (1990) | (2) R.F. Haseloff; FEBS Lett. 418, 73 (1997) | (3) R.A. Floyd; Adv. Pharmacol. 38, 361 (1997) | (4) T. Miyajima & Y. Kotake; Free Radic. Biol. Med. 22, 463 (1997) | (5) Y. Kotake, et al.; Biochim. Biophys. Acta 1448, 77 (1998) | (6) R.A. Floyd; Proc. Soc. Exp. Biol. Med. 222, 236 (1999) (Review) | (7) Y. Kotake; Antioxid. Redox Signal. 1, 481 (1999) (Review) | (8) E. Ho, et al.; Free Radic. Biol. Med. 28, 604 (2000) | (9) P. Kelicen, et al.; Neuroreport 13, 1057 (2002) | (10) W.N. Hassan, et al.; Free Radic. Biol. Med. 32, 551 (2002) | (11) K. Saito & H. Yoshioka; Free Radic. Res. 36, 143 (2002) | (12) R.A Floyd; Anticanc. Agents Med. Chem. 11, 373 (2011)
Related Products
| Product Name | Product Code | Supplier |
|---|