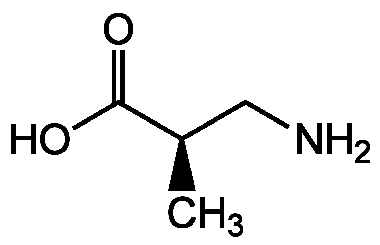
R-BAIBA
Product Code:
CDX-A0148
CDX-A0148
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-A0148-M100 | 100 mg | £194.00 |
Quantity:
| CDX-A0148-M500 | 500 mg | £560.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
D-3-Amino-isobutyric acid
Appearance:
White powder.
CAS:
2140-95-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H319
InChi:
InChI=1S/C4H9NO2/c1-3(2-5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m1/s1
InChiKey:
QCHPKSFMDHPSNR-GSVOUGTGSA-N
Long Description:
Chemical. CAS: 2140-95-6. Formula: C4H9NO2. MW: 103.12. Synthetic Beta-Aminoisobutyric acid is a non-protein amino acid originating from the catabolism of thymine and valine. beta-Aminoisobutyric acid occurs in two isomeric forms and both enantiomers of beta-Aminoisobutyric acid can be detected in human urine and plasma. In plasma, the S-enantiomer is the predominant type due to active renal reabsorption. In contrast, urine almost exclusively contains the R-enantiomer of beta-Aminoisobutyric acid, which is eliminated both by filtration and tubular secretion. The S-enantiomer of beta-Aminoisobutyric acid is predominantly derived from the catabolism of valine, the R-enantiomer is the product of the catabolism of the pyrimidine bases uracil and thymine by the enzyme dihydropyrimidine dehydrogenase (DPD), in what constitutes the first step of the pyrimidine degradation pathway. Transient high levels of beta-Aminoisobutyric acid have been observed under a variety of pathological conditions such as lead poisoning, starvation, in total body irradiation and in a number of malignancies. Recently R-/S-enantiomer mixtures have been shown to be browning inducer of white adipose tissue.
MDL:
MFCD01076229
Molecular Formula:
C4H9NO2
Molecular Weight:
103.12
Package Type:
Vial
Precautions:
P305, P351, P338
Product Description:
Beta-Aminoisobutyric acid is a non-protein amino acid originating from the catabolism of thymine and valine. beta-Aminoisobutyric acid occurs in two isomeric forms and both enantiomers of beta-Aminoisobutyric acid can be detected in human urine and plasma. In plasma, the S-enantiomer is the predominant type due to active renal reabsorption. In contrast, urine almost exclusively contains the R-enantiomer of beta-Aminoisobutyric acid, which is eliminated both by filtration and tubular secretion. The S-enantiomer of beta-Aminoisobutyric acid is predominantly derived from the catabolism of valine, the R-enantiomer is the product of the catabolism of the pyrimidine bases uracil and thymine by the enzyme dihydropyrimidine dehydrogenase (DPD), in what constitutes the first step of the pyrimidine degradation pathway. Transient high levels of beta-Aminoisobutyric acid have been observed under a variety of pathological conditions such as lead poisoning, starvation, in total body irradiation and in a number of malignancies. Recently R-/S-enantiomer mixtures have been shown to be browning inducer of white adipose tissue.
Purity:
>99% (TLC)
Signal word:
Warning
SMILES:
C[C@H](CN)C(O)=O
Solubility Chemicals:
Soluble in methanol or water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) S. Landaas & E. Solem; Scand. J. Clin. Lab. Invest. 43, 95 (1983) | (2) F. Podebrad, et al.; Clin. Chim. Acta 292, 93 (2000) | (3) A.B. Van Kuilenburg, et al.; Biochem. J. 379, 119 (2004) | (4) Z. Wang, et al. Commun. Biol. 3, 837 (2020) | (5) K. Awad, et al.; Int. J. Mol. Sci. 22, 497 (2021)
Related Products
| Product Name | Product Code | Supplier | S-BAIBA | CDX-A0147 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL-3-Hydroxyisobutyric acid sodium salt | CDX-H0085 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (E)-8-Methyl-6-nonenoic acid | CDX-M0209 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||