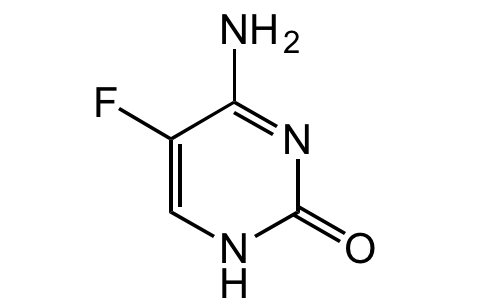
5-Fluorocytosine
Product Code:
CDX-F0077
CDX-F0077
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-F0077-G001 | 1 g | £157.00 |
Quantity:
| CDX-F0077-G005 | 5 g | £596.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
4-Amino-5-fluoro-2(1H)-pyrimidinone; Flucytosine; NSC 103805; Ancotil; 5-FC; Ancobon; Ro 2-9915
Appearance:
White to off-white powder.
CAS:
2022-85-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H361
InChi:
InChI=1S/C4H4FN3O/c5-2-1-7-4(9)8-3(2)6/h1H,(H3,6,7,8,9)
InChiKey:
XRECTZIEBJDKEO-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 2022-85-7. Formula: C4H4FN3O. MW: 129.09. Synthetic Fluorinated pyrimidine analog. Antimycotic prodrug that is converted by cytosine deaminase to 5-fluorouracil (a widely used cytotoxic drug) and further metabolized to fluorinated ribo- and deoxyribonucleotides. Inhibits DNA and RNA synthesis and interfers with ribosomal protein synthesis. Displays antifungal and antitumor activity. Lately with the development of gene therapy, it has been introduced as a prodrug in combination with the cytosine deaminase suicide gene as antitumor compound, significantly improving survival and reducing tumor size in selected tumors.
MDL:
MFCD00006035
Molecular Formula:
C4H4FN3O
Molecular Weight:
129.09
Package Type:
Vial
Precautions:
P281
Product Description:
Fluorinated pyrimidine analog. Antimycotic prodrug that is converted by cytosine deaminase to 5-fluorouracil (a widely used cytotoxic drug) and further metabolized to fluorinated ribo- and deoxyribonucleotides. Inhibits DNA and RNA synthesis and interfers with ribosomal protein synthesis. Displays antifungal and antitumor activity. Lately with the development of gene therapy, it has been introduced as a prodrug in combination with the cytosine deaminase suicide gene as antitumor compound, significantly improving survival and reducing tumor size in selected tumors.
Purity:
>99% (TLC)
Signal word:
Warning
SMILES:
NC1=NC(=O)NC=C1F
Solubility Chemicals:
Soluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) R.J. Holt & R.L. Newman; J. Clin. Pathol. 26, 167 (1973) | (2) R.B. Diasio, et al.; Biochem. Pharmacol. 27, 703 (1978) | (3) D.G. Osterman, et al.; Biochemistry 27, 5204 (1988) | (4) T. Ichikawa, et al.; Cancer Gene Ther. 7, 74 (2000) | (5) C. Fuerer & R. Iggo; Gene Ther. 11, 142 (2004) | (6) K. Kurozumi, et al.; J. Neurooncol. 66, 117 (2004)
Related Products
| Product Name | Product Code | Supplier | Piroctone olamine | CDX-P0125 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|