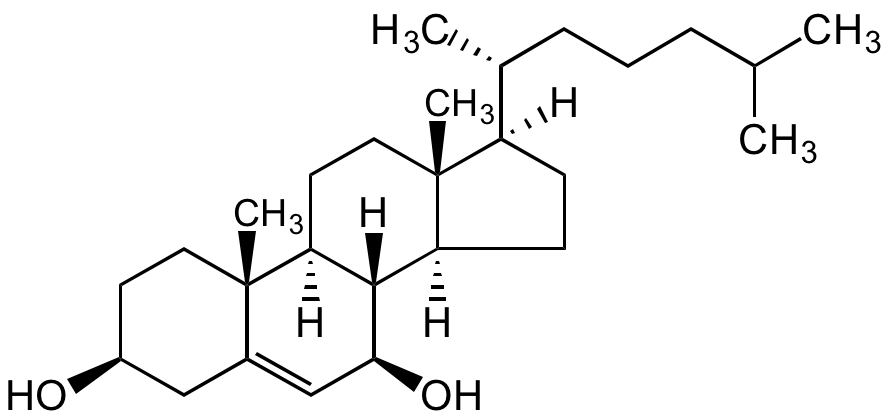
7beta-Hydroxycholesterol
Product Code:
CDX-H0116
CDX-H0116
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-H0116-M005 | 5 mg | £157.00 |
Quantity:
| CDX-H0116-M010 | 10 mg | £255.00 |
Quantity:
| CDX-H0116-M100 | 100 mg | £1,170.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
5-Cholestene-3beta,7beta-diol
Appearance:
White to white-yellow powder.
CAS:
566-27-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C27H46O2/c1-17(2)7-6-8-18(3)21-9-10-22-25-23(12-14-27(21,22)5)26(4)13-11-20(28)15-19(26)16-24(25)29/h16-18,20-25,28-29H,6-15H2,1-5H3/t18-,20+,21-,22+,23+,24+,25+,26+,27-/m1/s1
InChiKey:
OYXZMSRRJOYLLO-KGZHIOMZSA-N
Long Description:
Chemical. CAS: 566-27-8. Formula: C27H46O2. MW: 402.65. Synthetic Oxysterole. Cholesterol oxidation metabolite. Induces cell death/apoptosis and shows antiproliferative activity against cancer cells, involving mitochondrial perturbation, oxidative stress and lysosomal destabilization. Its membrane organizing properties may have implications in Alzheimer?s disease. Increased levels correlate with risk of cardiovascular diseases including atherosclerosis. Shown to have proinflammatory activity.
MDL:
MFCD00058406
Molecular Formula:
C27H46O2
Molecular Weight:
402.65
Package Type:
Vial
Product Description:
Oxysterole. Cholesterol oxidation metabolite. Induces cell death/apoptosis and shows antiproliferative activity against cancer cells, involving mitochondrial perturbation, oxidative stress and lysosomal destabilization. Its membrane organizing properties may have implications in Alzheimer?s disease. Increased levels correlate with risk of cardiovascular diseases including atherosclerosis. Shown to have proinflammatory activity.
Purity:
>98% (HPLC)
SMILES:
[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C
Solubility Chemicals:
Soluble in ethanol, chloroform or methanol.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) S. Lemaire, et al.; FEBS Lett. 440, 434 (1998) | (2) G. Lizard, et al.; Arterioscler. Thromb. Vasc. Biol. 19, 1190 (1999) | (3) J. Wang, et al.; Biochem. 43, 1010 (2004) | (4) S. Roussi, et al.; Cell Death Differ. 12, 128 (2005) | (5) K.A. Kang, et al.; Biol. Pharm. Bull. 28, 1377 (2005) | (6) S. Roussi, et al.; Apoptosis 12, 87 (2007) | (7) S. Lordan, et al.; Int. J. Toxicol. 27, 279 (2008) | (8) W. Li, et al.; Free Radic. Res. 43, 1072 (2009) | (9) L. Trevisi, et al.; J. Vasc. Res. 47, 241 (2010) | (10) C. Mascia, et al.; Free Radic. Biol. Med. 49, 2049 (2010)
Related Products
| Product Name | Product Code | Supplier | 7-Dehydrocholesterol | CDX-D0331 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7beta-Hydroxy-cholesteryl-bishemisuccinate-diethanolamine salt | CDX-H0123 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||