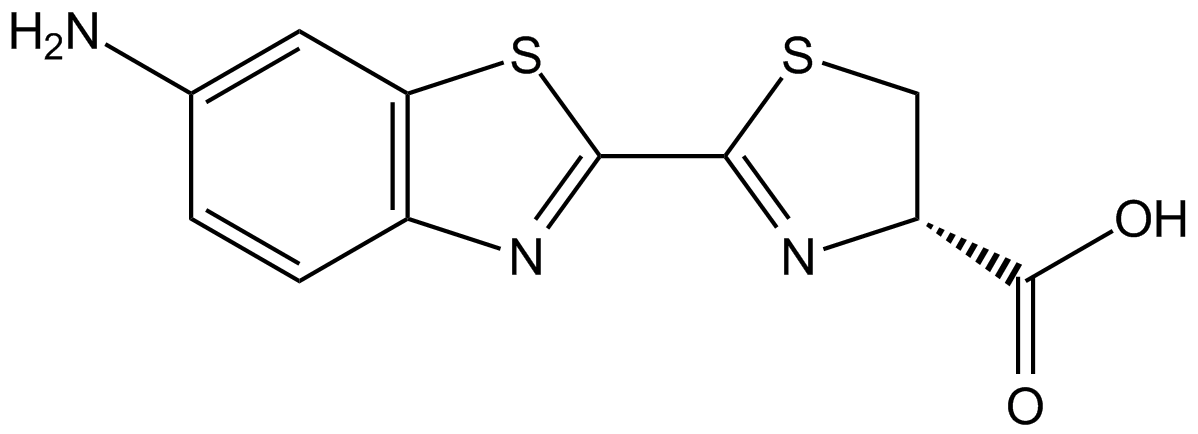
6-Amino-D-luciferin
Product Code:
CDX-A0306
CDX-A0306
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-A0306-M001 | 1 mg | £84.00 |
Quantity:
| CDX-A0306-M005 | 5 mg | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
4,5-Dihydro-2[6-amino-2-benzthiazolyl]-4-thiazole carboxylic acid
Appearance:
Yellow powder.
CAS:
161055-47-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light and moisture.
InChi:
InChI=1S/C11H9N3O2S2/c12-5-1-2-6-8(3-5)18-10(13-6)9-14-7(4-17-9)11(15)16/h1-3,7H,4,12H2,(H,15,16)/t7-/m1/s1
InChiKey:
HKSJKXOOBAVPKR-SSDOTTSWSA-N
Long Description:
Chemical. CAS: 161055-47-6. Formula: C11H9N3O2S2. MW: 279.34. Synthetic. Amino analog of the common D-luciferin substrate for use in measuring firefly luciferase activity. This cell permeable reagent can be used when considering design of proluminescent bioconjugates (peptidase substrates) of luciferin for ultrasensitive luminescent assays. In bioluminescent protease assays peptides or amino acids are conjugated to the luciferin analog 6-amino-D-luciferin. These substrates are useful for ultrasensitive analysis of a variety of protease enzymes including various caspases and cathepsins, chymotrypsin, trypsin, elastase, kallikrein, thrombin, ficin, bromoalain, plasmin, papain, ficin and many others or even for use in vivo tracing of luciferase transfected cells in living tissues. The resulting bioluminescence spectrum of reaction of 6-Amino-D-luciferin is very similar to that observed for the natural substrate D-luciferin. Peptidase assays using a bioluminogenic substrate can be easily adapted to 96-well formats for HTS-based assays. Using this principle, it is possible to design an assay to detect almost any peptidase or protease activity via bioluminescence by using a substrate with a suitable peptide sequence. Applications in pathogen detection, discovery of protease inhibitors, probing cell physiology and assessing protease activity in oncogenesis are possible at extraordinary sensitivity.
MDL:
MFCD00168449
Molecular Formula:
C11H9N3O2S2
Molecular Weight:
279.34
Package Type:
Vial
Product Description:
Amino analog of the common D-luciferin substrate for use in measuring firefly luciferase activity. This cell permeable reagent can be used when considering design of proluminescent bioconjugates (peptidase substrates) of luciferin for ultrasensitive luminescent assays. In bioluminescent protease assays peptides or amino acids are conjugated to the luciferin analog 6-amino-D-luciferin. These substrates are useful for ultrasensitive analysis of a variety of protease enzymes including various caspases and cathepsins, chymotrypsin, trypsin, elastase, kallikrein, thrombin, ficin, bromoalain, plasmin, papain, ficin and many others or even for use in vivo tracing of luciferase transfected cells in living tissues. The resulting bioluminescence spectrum of reaction of 6-Amino-D-luciferin is very similar to that observed for the natural substrate D-luciferin. Peptidase assays using a bioluminogenic substrate can be easily adapted to 96-well formats for HTS-based assays. Using this principle, it is possible to design an assay to detect almost any peptidase or protease activity via bioluminescence by using a substrate with a suitable peptide sequence. Applications in pathogen detection, discovery of protease inhibitors, probing cell physiology and assessing protease activity in oncogenesis are possible at extraordinary sensitivity.
Purity:
>99% (HPLC)
SMILES:
NC1=CC2=C(C=C1)N=C(S2)C1=N[C@H](CS1)C(O)=O
Solubility Chemicals:
Soluble in water.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) R. Shinde, et al.; Biochemistry 45, 11103 (2006) | (2) A. Dragulescu-Andrasi, et al.; Bioconjugate Chem. 20, 1660 (2009) | (3) H. Takakura, et al.; Chem. Asian J. 5, 2053 (2010) | (4) K.R. Harwood, et al.; Chem. Biol. 18, 1649 (2011) | (5) S.J. Orcutt, et al.; BBA Mol. Res. 1823, 2079 (2012) | (6) Z-S. Li, et al.; J. Photochem. Photobiol. A: Chem. 243, 7 (2012) | (7) R. Kojima, et al.; Angew. Chem. Int. Ed. 52, 1175 (2013) | (8) J. Li, et al.; Anal. Chem. 86, 2747 (2014) | (9) V.R. Viviani, et al.; Biochemistry 53, 5208 (2014) | (10) D.M. Mofford, et al.; JACS 136, 13277 (2014) | (11) Y. Nam, et al.; Chem. Commun. 52, 1128 (2016)
Related Products
| Product Name | Product Code | Supplier | 2',7'-Dichlorofluorescein | CDX-D0004 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N,N-Dimethyl-6-propionyl-2-naphthylamine | CDX-D0077 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4-[4'-(2'-Methyl)thiazolyl]phenol | CDX-M0006 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||