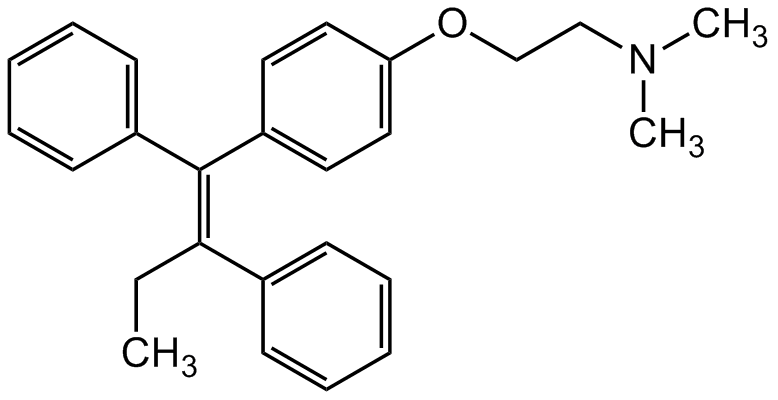
Tamoxifen
Product Code:
CDX-T0200
CDX-T0200
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short Term: +4°C. Long Term: +4°C
Short Term: +4°C. Long Term: +4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-T0200-G001 | 1 g | £72.00 |
Quantity:
| CDX-T0200-G005 | 5 g | £255.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
(Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene; trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine
Appearance:
White powder.
CAS:
10540-29-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Keep cool and dry.Protect from light and moisture.
Hazards:
H350, H360, H362
InChi:
InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25-
InChiKey:
NKANXQFJJICGDU-QPLCGJKRSA-N
Long Description:
Chemical. CAS: 10540-29-1. Formula: C26H29NO. MW: 371.51. Synthetic. Selective estrogen receptor modulator (SERM) used as adjuvant therapy for estrogen-dependent breast cancer. Antagonist of ER action in breast tissue and breast cancer cells and ER agonist in bone, uterus, and the cardiovasculature system. This prodrug is metabolized by cytochrome P450 (CYP450) enzymes to the active metabolites N-desmethyl-TMX, 4-hydroxy-N-desmethyl-TMX (endoxifen) and 4-hydroxy-TMX (afimoxifene). N,N-Didesmethyl-4-hydroxytamoxifen (norendoxifen), another active metabolite has been found to act as a potent competitive aromatase inhibitor and may also be involved in its antiestrogenic activity. Binds microsomal antiestrogen binding sites, altering cholesterol esterification at therapeutic doses and impacting breast cancer cell differentiation, apoptosis, and autophagy. Protein kinase C (PKC) inhibitor and anti-angiogenetic factor.
MDL:
MFCD00010454
Molecular Formula:
C26H29NO
Molecular Weight:
371.51
Package Type:
Vial
Precautions:
P201, P263, P308, P313
Product Description:
Selective estrogen receptor modulator (SERM) used as adjuvant therapy for estrogen-dependent breast cancer. Antagonist of ER action in breast tissue and breast cancer cells and ER agonist in bone, uterus, and the cardiovasculature system. This prodrug is metabolized by cytochrome P450 (CYP450) enzymes to the active metabolites N-desmethyl-TMX, 4-hydroxy-N-desmethyl-TMX (endoxifen) and 4-hydroxy-TMX (afimoxifene). N,N-Didesmethyl-4-hydroxytamoxifen (norendoxifen), another active metabolite has been found to act as a potent competitive aromatase inhibitor and may also be involved in its antiestrogenic activity. Binds microsomal antiestrogen binding sites, altering cholesterol esterification at therapeutic doses and impacting breast cancer cell differentiation, apoptosis, and autophagy. Protein kinase C (PKC) inhibitor and anti-angiogenetic factor.
Purity:
>98% (NMR)
Signal word:
Warning
SMILES:
CN(CCOC1=CC=C(/C(C2=CC=CC=C2)=C(C3=CC=CC=C3)/CC)C=C1)C
Solubility Chemicals:
Soluble in DMSO, ethanol, methanol, propylene or chloroform.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
Documents
References
(1) K. B. Horwitz & W. L. McGuire; J. Biol. Chem. 253, 8185 (1978) | (2) V.C. Jordan, et al.; Cancer Treat. Rep. 64, 745 (1980) | (3) C.A. O'Brian, et al.; Cancer Res. 45, 2462 (1985) | (4) K.J. Edwards, et al.; J. Med. Chem. 35, 2753 (1992) | (5) M. Clarke, et al.; Lancet 351, 1451 (1998) | (6) D.A. Tonetti & V.C. Jordan; Mol. Med. Today 2, 218 (1996) | (7) J.M. Hall, et al.; J. Biol. Chem. 276, 36869 (2001) | (8) P. Thomas, et al.; Endocrinol. 146, 624 (2005) | (9) M.S. Singh, et al.; Breast 20, 111 (2011) | (10) P. de Medina, et al.; Chem. Phys. Lip. 164, 432 (2011) | (11) V.K. Todorova, et al.; Cancer Chemother. Pharmacol. 67, 285 (2011)
Related Products
| Product Name | Product Code | Supplier | Tamoxifen citrate salt | CDX-T0201 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|