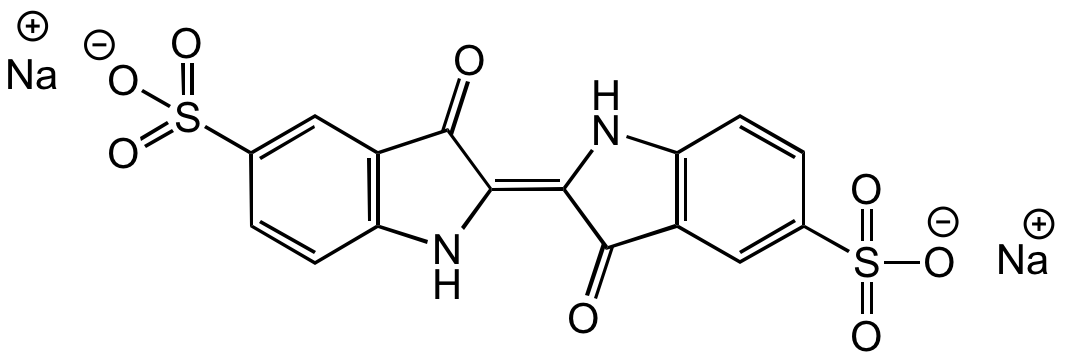
Indigo Carmine
Product Code:
CDX-I0063
CDX-I0063
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
Short Term: +20°C. Long Term: +20°C
Short Term: +20°C. Long Term: +20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-I0063-M025 | 25 mg | £108.00 |
Quantity:
| CDX-I0063-M100 | 100 mg | £304.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Acid Blue 74; Indigo-5,5'-disulfonic acid disodium salt; Indigocarmine; Amacid Brilliant Blue; CI 73015
Appearance:
Dark blue powder.
CAS:
860-22-0
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302
InChi:
InChI=1S/C16H10N2O8S2.2Na/c19-15-9-5-7(27(21,22)23)1-3-11(9)17-13(15)14-16(20)10-6-8(28(24,25)26)2-4-12(10)18-14;;/h1-6,17-18H,(H,21,22,23)(H,24,25,26);;/q;2*+1/p-2/b14-13+;;
InChiKey:
KHLVKKOJDHCJMG-QDBORUFSSA-L
Long Description:
Chemical. CAS: 860-22-0. Formula: C16H8N2Na2O8S2. MW: 466.35. Synthetic. Indigo carmine is a blue, anionic indigoid dye consisting of an indigo dimer with sodium salts of sulfonic acids at positions 5 and 5'. This compound has been used as a tissue stain, pH indicator (Blue (at pH11.5) to yellow (at pH14.0)) and tool for the determination of hypochlorite in solutions. It is also applied in detecting microorganisms, treating amyloidosis, in medical devices and drug delivery systems. It can be used as a contrast stain for plasma when red nuclear stains are used. It is widely used in food and cosmetic industry (beverages, chewing gums, candies, drinks, frozen products, sweetener, tablets, sunscreen, skin and hair products) and as an additive or as a dye for cationic cotton fabrics, filters, display devices, inks, toners, paints, photographic material and toys. Spectral Data: Absorption lambdamax 608nm.
MDL:
MFCD00005723
Molecular Formula:
C16H8N2Na2O8S2
Molecular Weight:
466.35
Package Type:
Vial
Precautions:
P270, P301, P312, P330
Product Description:
Indigo carmine is a blue, anionic indigoid dye consisting of an indigo dimer with sodium salts of sulfonic acids at positions 5 and 5'. This compound has been used as a tissue stain, pH indicator (Blue (at pH11.5) to yellow (at pH14.0)) and tool for the determination of hypochlorite in solutions. It is also applied in detecting microorganisms, treating amyloidosis, in medical devices and drug delivery systems. It can be used as a contrast stain for plasma when red nuclear stains are used. It is widely used in food and cosmetic industry (beverages, chewing gums, candies, drinks, frozen products, sweetener, tablets, sunscreen, skin and hair products) and as an additive or as a dye for cationic cotton fabrics, filters, display devices, inks, toners, paints, photographic material and toys. Spectral Data: Absorption lambdamax 608nm.
Purity:
>95% (UV/Vis)
Signal word:
Warning
SMILES:
O=C(C(C=C(S(=O)([O-])=O)C=C1)=C1N/2)C2=C3C(C(C=C(S(=O)([O-])=O)C=C4)=C4N3)=O.[Na+].[Na+]
Solubility Chemicals:
Soluble in water. Slightly soluble in ethanol. Insoluble in benzene or chloroform.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at +20°C.
Documents
References
(1) G.G. Reinle & E.S. Depuy; Cal. State J. Med. 18, 49 (1920) | (2) M. Mortreuil-Langlois; Stain Technol. 37, 175 (1962) | (3) K. Ikeda, et al.; Endoscopy 14, 119 (1982) | (4) F.C. Monson, et al.; J. Urol. 145, 842 (1991) | (5) M. Lee & R. Sharifi; Urology 47, 783 (1996) | (6) A. Harriram, et al.; J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 3, 1055 (2003) | (7) A.G. Prado, et al.; J. Colloid Interface Sci. 277, 43 (2004) | (8) D.P. Hurlstone, et al.; Biotech. Histochem. 82, 57 (2007) (Review) | (9) R.W. Sabnis; Handbook of biological dyes and stains (2010) | (10) B.E. Lee, et al.; BMC Gastroenterol. 10, 97 (2010) | (11) Y. Ma, et al. ; J. Agric. Food Chem. 60, 10867 (2012) | (12) M. Yao, et al.; Sci. Rep. 4, 3650 (2014) | (13) A.L. Costa, et al. ; Chemistry 21, 12069 (2015)
Related Products
| Product Name | Product Code | Supplier | DCFA | CDX-C0043 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHPDS | CDX-D0078 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fluorescein octadecyl ester | CDX-F0074 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HPTS | CDX-H0034 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8-Hydroxy-N,N,N',N',N'',N''-hexamethyl-pyrene-1,3,6-trisulfonamide | CDX-H0043 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1,3,6,8-Pyrenetetrasulfonic acid tetrasodium salt | CDX-P0021 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol red sodium salt | CDX-P0026 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SNARF-DE | CDX-S0037 | Chemodex | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||