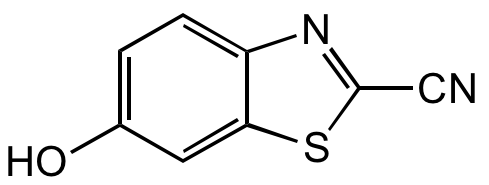
6-Hydroxybenzothiazole-2-carbonitrile
Product Code:
CDX-C0263
CDX-C0263
Regulatory Status:
RUO
RUO
Shipping:
Ambient
Ambient
Storage:
-20 °C
-20 °C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-C0263-G001 | 1 g | £108.00 |
Quantity:
| CDX-C0263-GG25 | 2.5 g | £218.00 |
Quantity:
Prices exclude any Taxes / VAT