The unique optical properties of gold nanoparticles make them very useful for bio-imaging. While immunogold labelling is the most common use of gold nanoparticles in this area, our supplier Nanoprobes offers a different imaging technology based on gold nanoparticles that allows selective and sensitive detection of recombinant proteins. Does this sound like something your research could benefit from? Read on!
Worth their weight in gold
Detection and imaging of tissues, cells and biomolecules is an important area of biomedicine where gold is earning a very strong reputation. The special optical properties of gold nanoparticles (AuNPs) that arise due to their Localised Surface Plasmon Resonance (LSPR), combined with their strong electron density and their optimal biocompatibility features, have positioned AuNPs high up in the list of indispensable tools for biomedical imaging.
The classic application of AuNPs in biological detection is as immune markers for electron microscopy (EM), commonly known as immunogold labelling. Because of the strong electron density of the AuNPs, these can be used to mark antibodies that will accurately locate antigens intracellularly by showing as clear and legible particles against the lower electron-dense background.1
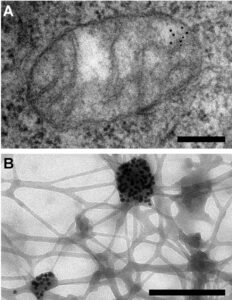
Figure 1. Electron microscopy reveals mitochondrial DNA in discrete foci. Bars: 200 nm. (A) Cytoplasmic section after immunogold labelling with anti-DNA; gold particles marking mtDNA are found near the mitochondrial membrane. (B) Whole mount view of cytoplasm after extraction with CSK buffer and immunogold labelling with anti-DNA; mtDNA (marked by gold particles) resists extraction. Iborra et al. BMC Biology 2004 2:9 doi:10.1186/1741-7007-2-9
Our supplier, Nanoprobes, has developed an alternative bio-imaging tool based on AuNPs that can be used for the detection of recombinant proteins, Ni-NTA-Nanogold®. This technology aims at imaging recombinant proteins by taking advantage of the ease of surface functionalisation of AuNPs and the high affinity between metals and what is known as His-tagged proteins.
His-tagged proteins and the Ni-NTA complex
A common procedure for the detection and purification of recombinant proteins is the use of tags. These tags are introduced in the recombinant protein of interest by adding a “tail” sequence coding for a string of amino acids into the genetic material encoding the protein.2
The His-tag is a popular genetic tag that is formed by 6 histidines. Histidines are known to have a high affinity for metals such as Ni2+, Co2+, Zn2+, Cu2+, Fe3+ and Mn2+, and this has fostered the use of these metals in the purification of His-tagged proteins by immobilized metal-ion affinity chromatography (IMAC).3 Ni-NTA (where NTA stands for tetradentate nitrilotriacetic acid) has been found to be the optimal complex for this application, where Ni2+ is able to bind to two histidines from the his-tagged protein (see Figure 2).
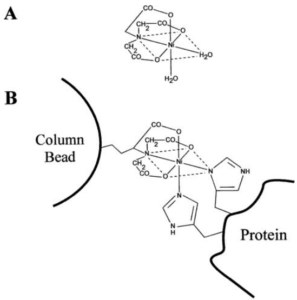 Figure 2. A) Complex formed between Ni2+ and NTA. NTA possess three carboxyl groups and one nitrogen to bind to Ni2+. Since Ni2+ has six coordination sites, its coordination with NTA leaves two sites free that are bound to two water molecules. B) Ni-NTA column showing the bonding to a His-tagged protein. The NTA is attached to the column matrix material, while the two water molecules bound to Ni2+ are replaced by the his-tagged protein. From reference 1.
Figure 2. A) Complex formed between Ni2+ and NTA. NTA possess three carboxyl groups and one nitrogen to bind to Ni2+. Since Ni2+ has six coordination sites, its coordination with NTA leaves two sites free that are bound to two water molecules. B) Ni-NTA column showing the bonding to a His-tagged protein. The NTA is attached to the column matrix material, while the two water molecules bound to Ni2+ are replaced by the his-tagged protein. From reference 1.
Apart from protein purification with IMAC, the ability of the Ni-NTA complex to strongly bind to his-tagged proteins is also useful for the localisation of recombinant proteins within cells.
Nanoprobes has combined the ability of the Ni-NTA complex to bind histidine with the excellent optical properties of gold nanoparticles to produce a new technology for the imaging of recombinant proteins, Ni-NTA-Nanogold®.
Ni-NTA-Nanogold®
Ni-NTA-Nanogold® consists of gold nanoparticles of 1.8, 5 or 10 nm in diameter which surfaces have been functionalised with ligands that are linked to Ni-NTA complexes. Each Ni2+ is complexed with one NTA, leaving two adjacent coordination sites that bind to two histidines from the His-tagged recombinant protein forming a stable complex (Figure 3). Strong binding is achieved when three adjacent Ni-NTA groups bind to a 6xHis-tag. The high electron density of gold means that Ni-NTA-Nanogold is able to label the his-tagged proteins it binds to, making them easily identifiable in EM.
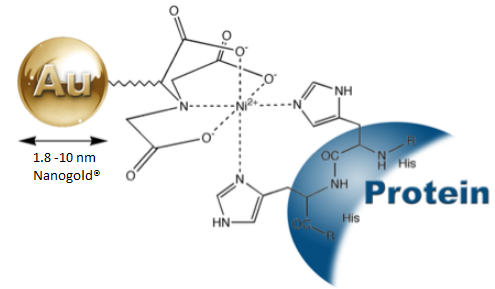 Figure 3. Labelling of his-tagged proteins with Ni-NTA-Nanogold®.
Figure 3. Labelling of his-tagged proteins with Ni-NTA-Nanogold®.
As previously mentioned, immunogold labelling is a popular approach in EM, however, the use of functionalised gold nanoparticles with the Ni-NTA complex provides the advantage of more precise localization of the His-tag sites, since no additional protein entities or antibodies are involved, and the distance from the gold particle surface to the His tag is less than 1.5 nm.3 Furthermore, Ni-NTA-Nanogold® bind to N-terminal, C-terminal and internal His-tag sequences, and recognize from five to ten consecutive histidine residues encoded by a variety of commercially available expression vectors.3
Hence, Ni-NTA-Nanogold® can be used to detect or localize 6xHistidine (His) or Poly-His tagged recombinant proteins without the use of anti-His-tag antibodies in protein mixtures, multisubunit protein complexes, tissue or cell samples. Furthermore, Ni-NTA-Nanogold can not only be used for imaging in EM but can also be used in light microscopy, blots and plate-based assays, and his-tagged proteins can be labelled under both non-denaturing and denaturing buffer conditions.4
Nanoprobes offers three Ni-NTA-Nanogold® products, 1.8 nm, 5 nm and 10 nm Ni-NTA-Nanogold® with 10 nm Ni-NTA-Nanogold® offering the optimum nanoparticle size for direct EM visualisation using low magnification without the need of silver/gold enhancement. Furthermore, 10 nm Ni-NTA-Nanogold® can also be directly visualized as pink bands on western blots and the LRSP band of gold nanoparticles can be measured using a spectrophotometer in microplate assays.4 10 nm Ni-NTA-Nanogold® can also be used to identify His-tagged proteins containing eluent fractions from Immobilized Metal Affinity Chromatography (IMAC) procedures using a simple dot blot procedure.4 Other advantages of using 10 nm Ni-NTA-Nanogold® are:
- High solubility, biocompatibility, and stability: 10 nm Ni-NTA-Nanogold® is prepared using a stable, highly hydrophilic surface functionalization.
- High penetration: 10 nm Ni-NTA-Gold is similar in size to an unlabeled antibody IgG molecule and much smaller than a 10 nm colloidal gold-antibody conjugate. It can therefore penetrate specimens and access sterically restricted interior sites much better than a 10 nm immunogold probe and provide higher labelling density. In some systems it may be used with stronger fixation or less permeabilization, enabling labelling with better ultrastructural preservation.
- Super sensitivity: the larger gold particle provides the highest sensitivity with virtually no background when used to detect His-tagged targets on blots.
If you think Ni-NTA-Nanogold® is something your research could benefit from and you would like more information about it, please don’t hesitate to contact us!
References
- Baltzer, N., Copponnex, T. Precious Metals for Biomedical Applications, Elsevier Science & Technology, 2014.
- Reddy,V., Lymar, E., Hu, M. and Hainfield, J.F. 5 nm Gold-Ni-NT binds His Tags https://www.nanoprobes.com/applications/MM05NTAGold.html
- Hainfield, J.F., Liu, W., Halsey, C.M.R., Freimuth, P., Powell, R.D. Ni–NTA–Gold Clusters Target His-Tagged Proteins. Journal of Structural Biology, vol. 127, pp. 185–198, 1999.
- 10 nm Ni-NTA-Nanogold® Product information. nanoprobes.com/pdf/Info_2084_v1_0_20170309.pdf