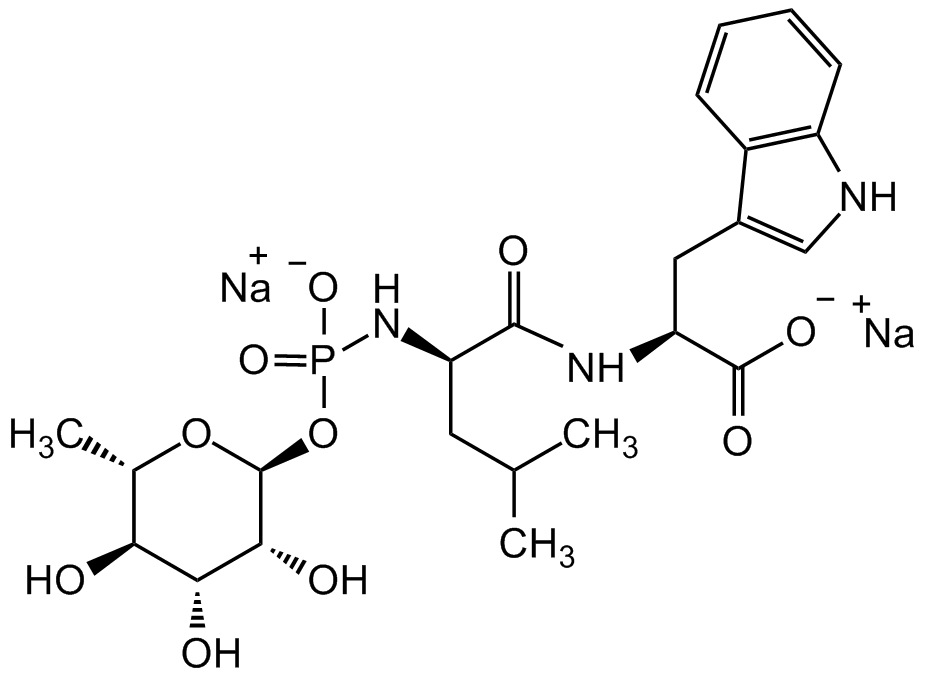
Phosphoramidon . disodium salt
| Code | Size | Price |
|---|
| AG-CP3-7008-M005 | 5 mg | £100.00 |
Quantity:
| AG-CP3-7008-M025 | 25 mg | £370.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term Storage: +4?C. Long Term Storage: -20?C
Images
Documents
Further Information
Alternate Names/Synonyms:
N-[N-[[(6-Deoxy-alpha-L-mannopyranosyl)oxy]hydroxyphosphinyl]-L-leucyl]-L-tryptophan disodium salt; NSC 694280
Appearance:
White solid.
CAS:
164204-38-0
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C23H34N3O10P.2Na/c1-11(2)8-16(26-37(33,34)36-23-20(29)19(28)18(27)12(3)35-23)21(30)25-17(22(31)32)9-13-10-24-15-7-5-4-6-14(13)15;;/h4-7,10-12,16-20,23-24,27-29H,8-9H2,1-3H3,(H,25,30)(H,31,32)(H2,26,33,34);;/q;2*+1/p-2/t12-,16+,17-,18-,19+,20+,23-;;/m0./s1
InChiKey:
OQKHVXFOYFBMDJ-FXPXQDEJSA-L4
Long Description:
Chemical. CAS: 164204-38-0. Formula: C23H32N3O10P . 2Na. Molecular Weight: 541.5 . 46.0. Phosphoramidon is a potent, reversible and competitive inhibitor of multiple soluble and membrane-bound zinc metalloproteases, particularly gluzincins, such as neutral endopeptidase NEP (Neprilysin; CD10; EC 3.4.24.11) (Ki = 2nM), NEP2 (Neprilysin-2) (Ki ~ 2nM), or endothelin-converting enzyme (ECE) (IC50 = 0.68?M). It inhibits the thermostable neutral metalloproteinase enzyme thermolysin and the integral membrane protein zinc metalloprotease ZMPSTE24. It has little or no effect on a variety of other proteases, including trypsin, papain, chymotrypsin, pepsin and angiotensin-converting enzyme. Shows anti-inflammatory, anti-neurodegenerative and cardioprotective properties. Blocks through NEP degradation of amyloid beta peptides and increases Abeta levels in rodents, indicating that abnormal amyloid processing in Alzheimer's disease may be carried out by a metalloendopeptidase. Plays also a role in the regulation of neuropeptide signaling and in the metabolism of bioactive peptides, such as bradykinin, neurotensin and enkephalin, via plasma membranes. Blocks the formation of endothelin-1, a proinflammatory mediator implicated in the pathogenesis of a variety of lung diseases.
MDL:
MFCD00077870
Molecular Formula:
C23H32N3O10P . 2Na
Molecular Weight:
541.5 . 46.0
Package Type:
Vial
Product Description:
Phosphoramidon is a potent, reversible and competitive inhibitor of multiple soluble and membrane-bound zinc metalloproteases, particularly gluzincins, such as neutral endopeptidase NEP (Neprilysin; CD10; EC 3.4.24.11) (Ki = 2nM), NEP2 (Neprilysin-2) (Ki ~ 2nM), or endothelin-converting enzyme (ECE) (IC50 = 0.68?M). It inhibits the thermostable neutral metalloproteinase enzyme thermolysin and the integral membrane protein zinc metalloprotease ZMPSTE24. It has little or no effect on a variety of other proteases, including trypsin, papain, chymotrypsin, pepsin and angiotensin-converting enzyme. Shows anti-inflammatory, anti-neurodegenerative and cardioprotective properties. Blocks through NEP degradation of amyloid beta peptides and increases Abeta levels in rodents, indicating that abnormal amyloid processing in Alzheimer's disease may be carried out by a metalloendopeptidase. Plays also a role in the regulation of neuropeptide signaling and in the metabolism of bioactive peptides, such as bradykinin, neurotensin and enkephalin, via plasma membranes. Blocks the formation of endothelin-1, a proinflammatory mediator implicated in the pathogenesis of a variety of lung diseases.
Purity:
>98% (HPLC)
SMILES:
C[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](OP(N[C@H](CC(C)C)C(N[C@@H](CC2=CNC3=C2C=CC=C3)C([O-])=O)=O)([O-])=O)O1.[Na+].[Na+]
Solubility Chemicals:
Soluble in water (25mg/ml) or DMSO (10mg/ml).
Source / Host:
Synthetic. Originally isolated from Streptomyces tanashiensis.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20?C.
References
Letter: A thermolysin inhibitor produced by Actinomycetes: phospholamidon: H. Suda, et al.; J. Antibiot. (Tokyo) 26, 621 (1973) | Phosphoramidon, a metalloproteinase inhibitor, suppresses the hypertensive effect of big endothelin-1: Y. Matsumura, et al.; Eur. J. Pharmacol. 185, 103 (1990) | Neutral endopeptidase-24.11 inhibitors: from analgesics to antihypertensives?: B.P. Roques & A. Beaumont; Trends Pharmacol. Sci. 11, 245 (1990) | Regional differences in endothelin converting enzyme activity in rat brain: inhibition by phosphoramidon and EDTA: T.D. Warner; Br. J. Pharmacol. 106, 948 (1992) | Molecular pharmacology of endothelin converting enzymes: A.J. Turner & L.J. Murphy; Biochem. Pharmacol. 51, 91 (1996) | Neutral endopeptidase and angiotensin-converting enzyme inhibitors increase nitric oxide production in isolated canine coronary microvessels by a kinin-dependent mechanism: X. Zhang, et al.; J. Cardiovasc. Pharmacol. 31, 623 (1998) | Soluble human endothelin-converting enzyme-1: expression, purification, and demonstration of pronounced pH sensitivity: K. Ahn, et al.; Arch. Biochem. Biophys. 359, 258 (1998) | Neurotensin is metabolized by endogenous proteases in prostate cancer cell lines: T.W. Moody, et al.; Peptides 19, 253 (1998) | Cell-specific activity of neprilysin 2 isoforms and enzymic specificity compared with neprilysin: C. Rose, et al.; Biochem. J. 363, 697 (2002) | Cardioprotective effects of phosphoramidon on myocardial structure and function in murine Chagas' disease: L.A. Jelicks, et al.; Int. J. Parasitol. 32, 1497 (2002) | Human neprilysin-2 (NEP2) and NEP display distinct subcellular localisations and substrate preferences: A.R. Whyteside & A.J. Turner; FEBS Lett. 582, 2382 (2008) | Phosphoramidon, an endothelin-converting enzyme inhibitor, attenuates lipopolysaccharide-induced acute lung injury: T.M. Bhavsar, et al.; Exp. Lung Res. 34, 141 (2008) | Amyloid-beta and Alzheimer?s disease: the role of neprilysin-2 in amyloid-beta clearance: R.A. Marr & D.M. Hafez; Front. Aging Neurosci. 6, 187 (2014)