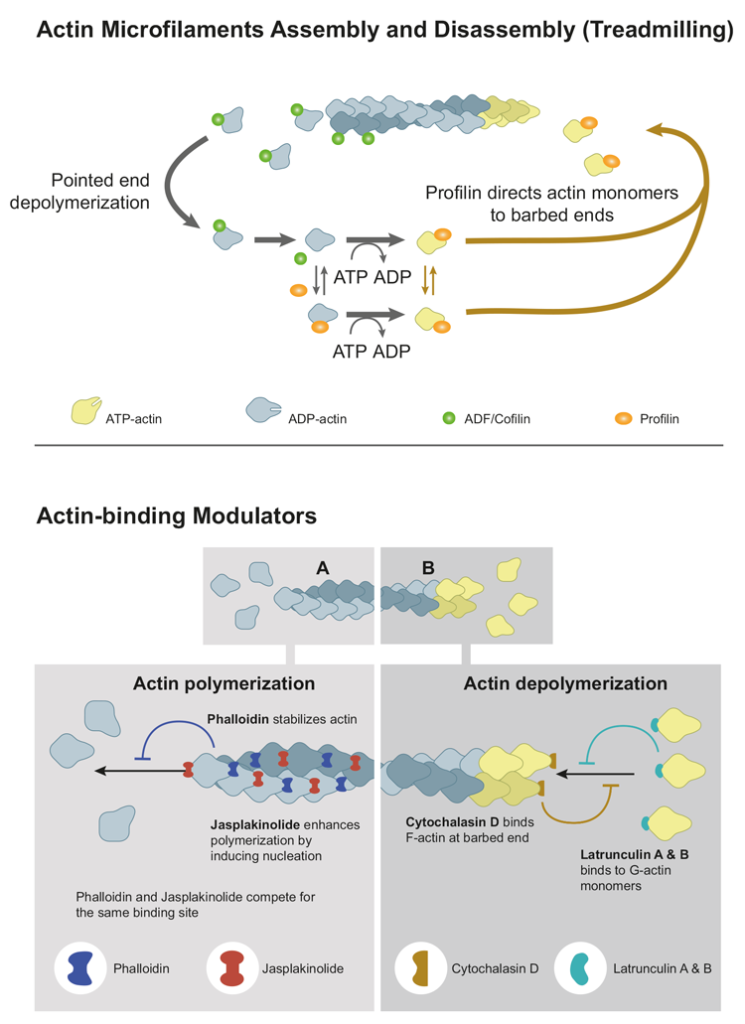
Microfilaments, also known as actin filaments, are one of the three main types of cytoskeletal filaments found within eukaryotic cells. They are composed of globular protein subunits called actin monomers that polymerise to form long, thin fibres. Actin must be bound to ATP to assemble into a polymer. The actin filament itself has structural polarity similar to microtubules. There are two distinct ends to the filament, called the “(-)” end and the “(+)” end. At the “(+)” end, actin subunits are added onto the elongating filament, and at the “(-)” end, actin subunits are disassembled. The ability of the actin filament to assemble and disassemble, thereby changing its length, is termed dynamic instability. This dynamic instability is integral to some cellular functions. The dynamic assembly and disassembly of microfilaments are regulated by a variety of proteins, including actin-binding proteins. These proteins control the polymerisation and depolymerisation of actin monomers, allowing cells to rapidly reorganise their cytoskeleton in response to different stimuli.
Microfilaments play a crucial role in i) the maintenance of the cell’s shape and structural integrity; ii) in cell motility, which includes processes like cell crawling, amoeboid movement and muscle contraction; iii) in cytokinesis, the process that divides the cell’s cytoplasm into two daughter cells; iv) in cell adhesion by interacting with integrin proteins in the cell membrane; v) in intracellular transport of organelles and vesicles; vi) in endocytosis and exocytosis, being involved in vesicle formation, movement, and fusion with the cell membrane; and vii) in signal transduction, transmitting mechanical signals within the cell, which can impact various cellular responses, such as gene expression and cell growth.
Latrunculin A & B
Latrunculins (including Latrunculin A & B) are natural compounds and a class of toxins originally isolated from the marine sponge Latrunculia magnifica. These compounds have been found to exhibit potent effects on actin polymerisation, which is a crucial process for maintaining the cell’s structural integrity, cell motility, cell division and other cellular functions. Latrunculins bind to actin monomers in a 1:1 ratio and prevent them from polymerising into filaments. This leads to the disassembly of existing actin filaments and the inhibition of new filament formation. Similar to cytochalasins, latrunculins are well-known cell-disrupting agents.
Latrunculins are used as research tools in cell biology and molecular studies to investigate the roles of actin in various cellular processes. Additionally, these compounds have shown potential in cancer research due to their ability to inhibit cell migration and invasion, which are processes associated with the metastasis of cancer cells.
Literature References:
- Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells: I. Spector, et al.; Science 219, 493 (1983)
- Latrunculin A is a potent inhibitor of phagocytosis by macrophages: C.A. de Oliveira & B. Mantovani; Life Sci. 43, 1825 (1988)
- Use of latrunculin-A, an actin monomer-binding drug: K. Ayscough; Methods Enzymol. 298, 18 (1998)
- Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A: E.G. Yarmola, et al.; J. Biol. Chem. 275, 28120 (2000)
- Latrunculin A has a strong anticancer effect in a peritoneal dissemination model of human gastric cancer in mice: H. Konishi, et al.; Anticancer Res. 29, 2091 (2009)
- Inhibition of interdomain motion in g-actin by the natural product latrunculin: a molecular dynamics study: S. Rennebaum & A. Caflisch; Proteins 80, 1998 (2012)
- Latrunculin A – Insight into Specific Modifications to Design Novel Drugs to Overcome Resistance: R. Lalitha, et al.; Curr. Comput. Aided Drug Dev. 12, 107 (2016)
- Polymerization and depolymerization of actin with nucleotide states at filament ends: I. Fujiwara, et al.; Biophys. Rev. 10, 1513 (2018)
- Discovery of cytotoxic natural products from Red Sea sponges: Structure and synthesis: S. Khan, et al.; Eur. J. Med. Chem. 220, 113491 (2021)
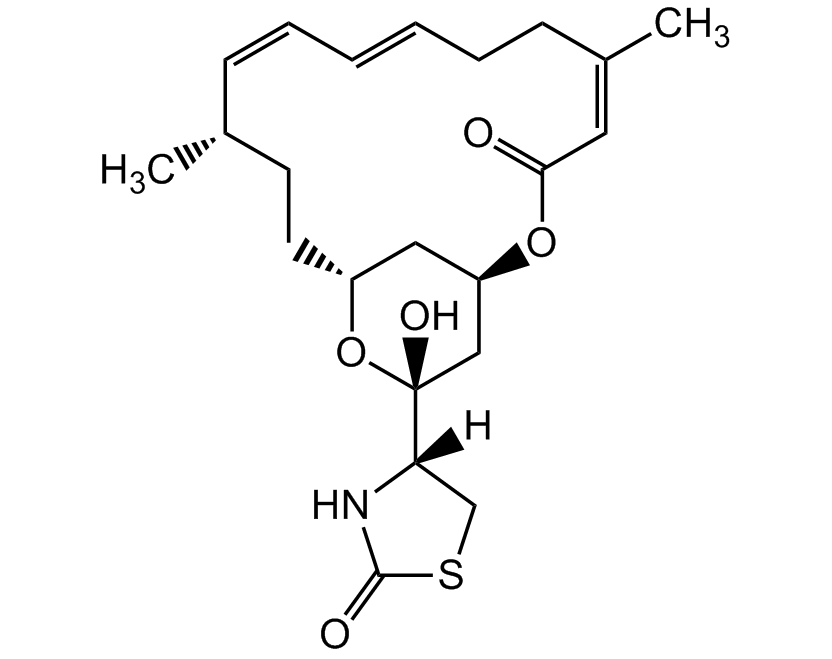
Latrunculin A
Latrunculin A is a cell permeable marine toxin, that disrupts microfilament-mediated processes. It is an actin polymerisation inhibitor in vitro and in vivo by the formation of a 1:1 complex with monomeric G-actin and it depolymerises actin filaments (F-actin). It is used as a potent phagocytosis inhibitor and anticancer compound, inhibiting tumour cell invasion.
AG-CN2-0027 (100 µg, 500 µg and BULK)
AdipoGen Life Sciences is an original Manufacturer of Latrunculin A. BULK quantities are available from Stock!
Product Specifications:
CAS: 76343-93-6
Source: Isolated from Latrunculia magnifica
Purity: >97% HPLC
Identity: Determined by 1H-NMR
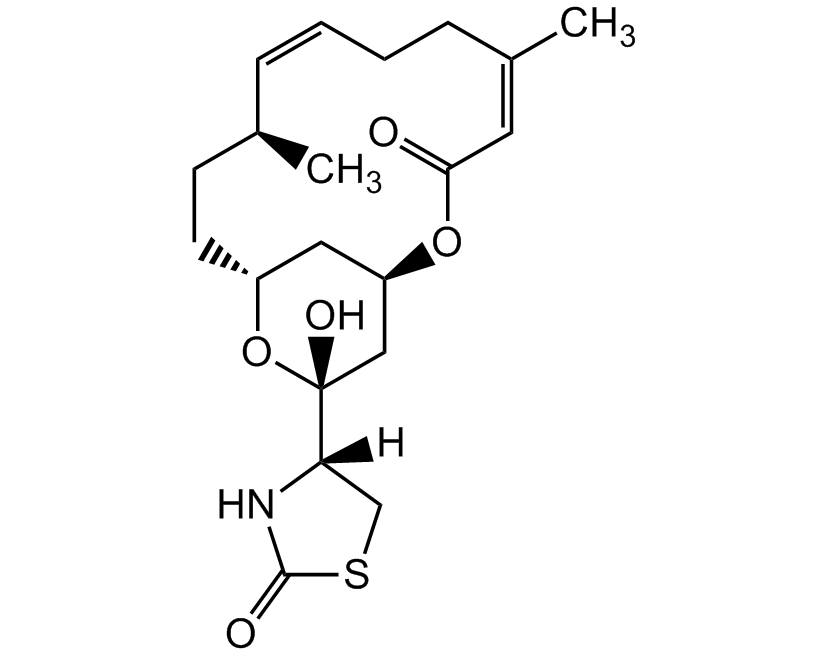
Latrunculin B
Latrunculin B is a cell permeable marine toxin, that disrupts microfilament-mediated processes. It is an actin polymerisation inhibitor in vitro and in vivo by the formation of a 1:1 complex with monomeric G-actin and it depolymerises actin filaments (F-actin). It is less potent than latrunculin A and may have fewer unwanted effects than latrunculin A.
AG-CN2-0031 (500 µg, 1 mg and BULK)
AdipoGen Life Sciences is the Key Manufacturer of Latrunculin B. BULK quantities are available from Stock!
Product Specifications:
CAS: 76343-94-7
Source: Isolated from Latrunculia magnifica
Purity: >98% HPLC
Identity: Determined by 1H-NMR
Originally posted by Adipogen on: https://adipogen.com/latrunculins-actin-polymerization
Caltag Medsystems is the distributor of Adipogen products in the UK and Ireland. If you have any questions about these products, please contact us.
