In 1986, the first monoclonal antibody became licensed as a therapeutic drug–an event occurring nine years after the generation of the first monoclonal antibody (mAb) [1]. The journey of how monoclonal antibodies went from lab discovery to lifesaving medicine is filled with scientific creativity and determination. These Y-shaped proteins, secreted by B cells, are made to hunt down specific invaders as part of the immune system.
Monoclonal antibody research changed dramatically in 1975 when Köhler and Milstein introduced the hybridoma technique. This process created “hybridomas” that could crank out large quantities of pure antibodies directed against a single target. Their technique was a giant leap forward because now, instead of isolating antibodies from the blood, scientists could grow hybridoma cells in the lab and collect the antibodies. This breakthrough unlocked the door to using monoclonal antibodies as therapies (2). Their innovation allowed for mass-producing targeted antibodies, which laid the groundwork for the many monoclonal antibody medicines used in clinics today (3).
What Are Monoclonal Antibodies?
Monoclonal antibodies are antibodies produced from a B-lymphocyte clone designed to bind to a specific epitope or antigen. Scientists use monoclonal antibodies in many therapeutic research areas, including oncology, haematology, ophthalmology, infectious diseases, transplantation, cardiovascular diseases, and auto-immune conditions such as rheumatoid arthritis and Crohn’s disease (1).
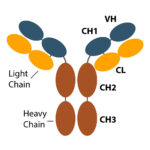
Typically, large molecules (~150 kDa), mAbs exhibit a complex protein structure (1). Purified from living cells, manufacturing monoclonal antibodies requires a multi-step, controlled process to ensure consistency. This allows limited batch-to-batch variability and limits deleterious modifications such as excess glycosylation and other post-translational modifications that could render the molecule ineffective or toxic.
Application of Monoclonal Antibodies in Research
Researchers use research-grade biosimilars, research mAbs mimicking therapeutic mAbs, as tools to explore the biological effects of these therapeutics further, research novel new therapies, and uncover effective off-target applications. With known and approved data on toxicity and efficacy, researchers use these biologics as benchmarks in their studies, allowing them to accelerate their findings from bench to clinic.
The scientific world has yet to un-tap the full potential of antibodies as therapies. As it stands, there are hundreds of phase III trials investigating various uses of antibodies in cancer, neurodegenerative diseases, auto-immune disorders, and many other conditions. Given the numerous ways mAbs are utilised in the clinic, one can expect their applications and benefits to continue expanding in the years to come. Traditional manufacturing technologies are being optimised, and the introduction of novel molecules, such as single-domain antibodies developed from camelids, is also pushing discoveries forward. Research-grade biosimilars further accelerate therapeutic antibody research by providing access to these reagents without the need to source therapeutic-grade biologics.
With more than 800 research-grade biosimilar antibodies in ProSci’s catalogue, these unique tools are available for researchers worldwide. Complimenting ProSci’s catalogue is a team of custom antibody specialists that provide expertise in the design, development, purification, and production of antibodies.
Research Grade Biosimilar Antibodies Available in the following Research Areas:
- Asthma
- Autoimmune Disorders – Rheumatoid Arthritis, Crohn’s, Diabetes
- Cancer
- Infection
- Inflammatory Diseases
- Neurodegenerative Disease
- Pain
References
- Liu JK. The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann Med Surg (Lond). 2014 Sep 11;3(4):113-6. doi: 10.1016/j.amsu.2014.09.001. PMID: 25568796; PMCID: PMC4284445.
- Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020 Jan 2;27(1):1. doi: 10.1186/s12929-019-0592-z. PMID: 31894001; PMCID: PMC6939334.
- Mahler S. Safety of biologics therapy: Monoclonal antibodies, cytokines, fusion proteins, hormones, enzymes, coagulation proteins, vaccines, botulinum toxins. MAbs. 2017 Aug/Sep;9(6):885-888. doi: 10.1080/19420862.2017.1343709. Epub 2017 Jul 5. PMID: 28678617; PMCID: PMC5540109.
Article developed in conjunction with Frieda Wiley, PharmD, MedVon Media Consulting.
Originally published by ProSci on: https://prosci-services.com/monoclonal-antibodies-biosimilars-in-research/?utm_source=ActiveCampaign&utm_medium=email&utm_content=ProSci+Blog%3A+%22The+Role+of+Monoclonal+Antibodies+%26+Biosimilars%22&utm_campaign=ProSci+Blog+%22The+Role+of+Monoclonal+Antibodies+%26+Biosimilars%22
Caltag Medsystems is the distributor of ProSci products in the UK and Ireland. If you have any questions about these products, please contact us.
