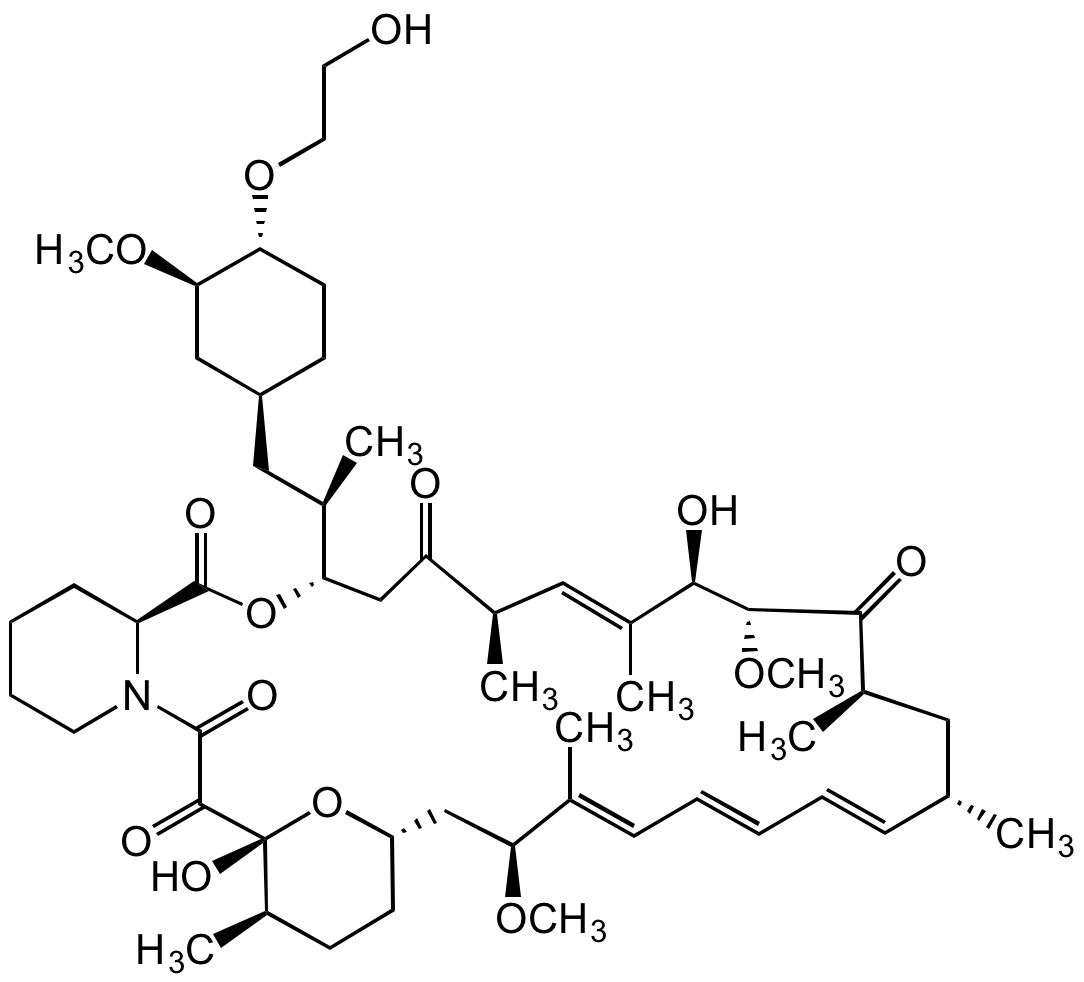
Everolimus Solution
| Code | Size | Price |
|---|
| CDX-E0574-M001 | 1 ml | £194.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
42-O-(2-Hydroxyethyl)rapamycin; NVP-RAD001; RAD001; SDZRAD; Zortress; Afinitor; Certican; Votubia
Appearance:
Liquid. 1mg/ml in acetonitrile.
CAS:
159351-69-6
Class:
3
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H225, H302+H332, H319
InChi:
InChI=1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44?,45+,46?,48-,49?,53-/m1/s1
InChiKey:
HKVAMNSJSFKALM-QATPHWSPSA-N
Long Description:
Chemical. CAS: 159351-69-6. Formula: C53H83NO14. MW: 958.22. Macrolide antibiotic, inhibiting bacterial protein synthesis. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP-12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to Rapamycin. Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo. This compound can be used as a reference material.
MDL:
MFCD00929329
Molecular Formula:
C53H83NO14
Molecular Weight:
958.22
Package Type:
Vial
PG:
II
Precautions:
P210, P305+P351+P338
Product Description:
Macrolide antibiotic, inhibiting bacterial protein synthesis. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP-12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to Rapamycin. Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo. This compound can be used as a reference material.
Purity:
>99% (HPLC)
Signal Word:
Danger
SMILES:
OCCO[C@H]1[C@H](OC)C[C@H](C[C@@H](C)[C@H](CC([C@H](C)/C=C(C)/[C@@H](O)[C@H]2OC)=O)OC([C@@H]3CCCCN3C(C([C@@]4(O)[C@H](C)CC[C@@H](C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C2=O)O4)=O)=O)=O)CC1
Transportation:
Excepted Quantity
UN Nummer:
1648
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) H.J. Schuurman, et al.; Transplantation 64, 32 (1997) | (2) W. Schuler, et al.; Transplantation 64, 36 (1997) | (3) P. Neuhaus, et al.; Liver Transpl. 7, 473 (2001) (Review) | (4) B. Nashan; Expert Opin. Investig. Drugs. 11, 1845 (2002) (Review) | (5) I. Beuvink, et al.; Cell 120, 747 (2005) | (6) J.K. Patel & J.A. Kobashigawa; Expert Opin. Pharmacother. 7, 1347 (2006) (Review) | (7) P. Smolewski; Anticancer Drugs 17, 487 (2006) (Review) | (8) K. Zitzmann, et al.; Neuroendocrinology 85, 54 (2007) | (9) Z. Zeng, et al.; Blood 109, 3509 (2007) | (10) R. Bianco, et al.; Br. J. Cancer 98, 923 (2008) | (11) A.I. Sanchez-Fructuoso; Expert Opin. Drug Metab. Toxicol. 4, 807 (2008) (Review) | (12) H.A. Lane, et al.; Clin. Cancer Res. 15, 1612 (2009) | (13) D. Lebwohl, et al.; Ann. N. Y. Acad. Sci. 1291, 14 (2013) (Review) | (14) U. Saran, et al.; Clin. Sci. 129, 895 (2015) (Review) | (15) Morviducci, et al.; Diabetes Res. Clin. Pract. (Epub ahead of print) (2018) (Review)