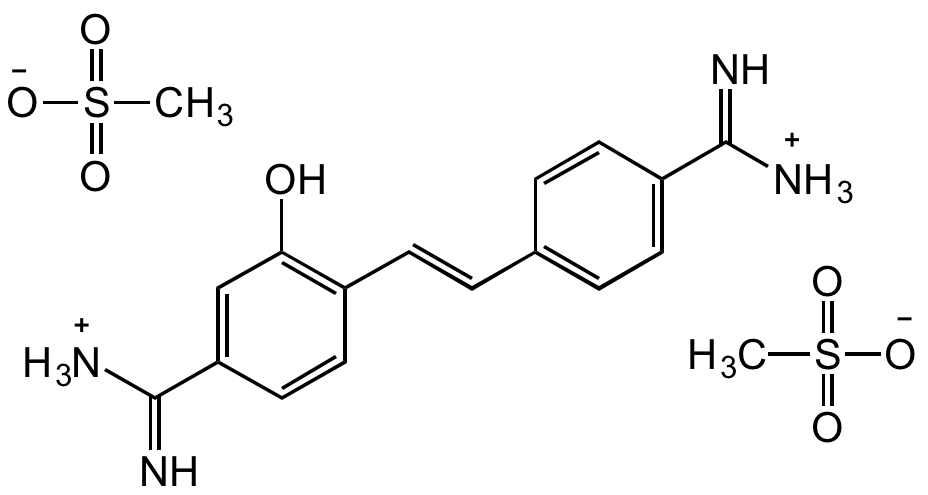
Hydroxystilbamidine bis(methanesulfonate)
| Code | Size | Price |
|---|
| CDX-H0100-M010 | 10 mg | £267.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-Hydroxystilbene-4,4'-dicarboxamidine bis(methanesulfonate); Fluoro Gold
Appearance:
Yellow solid.
CAS:
223769-64-0
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H315, H319, H335
InChi:
InChI=1S/C16H16N4O.2CH4O3S/c17-15(18)12-5-2-10(3-6-12)1-4-11-7-8-13(16(19)20)9-14(11)21;2*1-5(2,3)4/h1-9,21H,(H3,17,18)(H3,19,20);2*1H3,(H,2,3,4)/b4-1+;;
InChiKey:
YGNSQKCULHSJDC-HFPMQDOPSA-N
Long Description:
Chemical. CAS: 223769-64-0. Formula: C16H16N4O . 2CH4O3S. MW: 280.3 . 192.2. Synthetic. Hydroxystilbamidine (also called Fluoro Gold) is a cationic dye used for staining DNA and RNA. It exhibits distinctively different fluorescence emission profiles when bound to DNA compared to RNA. It is also frequently used as a retrograde neuronal tracer. The fluorescent tracer provides intense retrograde labeling that is extremely sensitive and reliable, does not diffuse out of retrograde-labeled neurons, and can be pressure-injected or introduced by iontophoresis into cells. Hydroxystilbamidine is compatible with most frequently used neuroanatomical techniques such as immunofluorescence, immunocytochemistry, autoradiography, and horseradish peroxidase-based histochemistry, paraffin embedding. Gold color is emitted when tissue is processed with a neutral pH buffer while blue/bright white is emitted when an acidic pH buffer is used. Spectral data: lambdaex=361nm and lambdaem=536nm. In addition, hydroxystilbamidine is an amidine antibiotic with antibacterial antifungal, antitrypanosomal, antimalarial and carcinostatic activities. Binds to DNA and RNA in a non-intercalating manner, and is a powerful inhibitor of ribonucleases. Binds also to lysosomes.
MDL:
MFCD01863083
Molecular Formula:
C16H16N4O . 2CH4O3S
Molecular Weight:
280.3 . 192.2
Package Type:
Vial
Precautions:
P261, P305+P351+P338
Product Description:
Hydroxystilbamidine (also called Fluoro Gold) is a cationic dye used for staining DNA and RNA. It exhibits distinctively different fluorescence emission profiles when bound to DNA compared to RNA. It is also frequently used as a retrograde neuronal tracer. The fluorescent tracer provides intense retrograde labeling that is extremely sensitive and reliable, does not diffuse out of retrograde-labeled neurons, and can be pressure-injected or introduced by iontophoresis into cells. Hydroxystilbamidine is compatible with most frequently used neuroanatomical techniques such as immunofluorescence, immunocytochemistry, autoradiography, and horseradish peroxidase-based histochemistry, paraffin embedding. Gold color is emitted when tissue is processed with a neutral pH buffer while blue/bright white is emitted when an acidic pH buffer is used. Spectral data: lambdaex=361nm and lambdaem=536nm. In addition, hydroxystilbamidine is an amidine antibiotic with antibacterial antifungal, antitrypanosomal, antimalarial and carcinostatic activities. Binds to DNA and RNA in a non-intercalating manner, and is a powerful inhibitor of ribonucleases. Binds also to lysosomes.
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
OC1=C(/C=C/C2=CC=C(C([NH3+])=N)C=C2)C=CC(C([NH3+])=N)=C1.CS(=O)([O-])=O.CS(=O)([O-])=O
Solubility Chemicals:
Soluble in water or DMSO (20mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) C.L. Taschdjian; J. Invest. Dermatol. 22, 521 (1954) | (2) E. Delain, et al.; J. Ultrastruct. Res. 37, 200 (1971) | (3) B. Festy & M. Daune; Biochemistry 12, 4827 (1973) | (4) P.M. Lizardi; J. Cell Biol. 87, 292 (1980) | (5) L.B. Murgatroyd; Histochemistry 74, 107 (1982) | (6) L.B. Murgatroyd; Diagn. Histopathol. 5, 219 (1982) | (7) L.C. Schmued & J.H. Fallon; Brain Res. 377, 147 (1986) | (8) L.C. Schmued, et al.; J. Neurocytol. 18, 333 (1989) | (9) L.C. Schmued & L. Heimer; J. Histochem. Cytochem. 38, 721 (1990) | (10) M.W. Wessendorf; Brain Res 553, 135 (1991) | (11) J.F. Bowyer, et al.; J. Pharmacol. Exp. Ther. 266, 1066 (1993) | (12) A. Puigdellivol-Sanchez, et al.; J. Neurosci. Method. 86, 7 (1998) | (13) L.A. Catapano, et al.; Methods Mol. Biol. 438, 353 (2008)