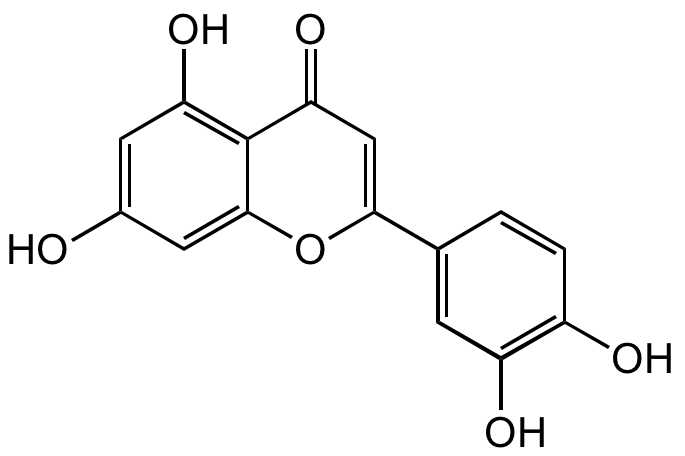
Luteolin
| Code | Size | Price |
|---|
| CDX-L0087-M010 | 10 mg | £48.00 |
Quantity:
| CDX-L0087-M050 | 50 mg | £102.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
3',4',5,7-Tetrahydroxyflavone; Luteolol; BRN 0292084; C.I. Natural Yellow 2; Digitoflavone; Flacitran; Flavopurpol; Daphneflavonol; Argemexitin
Appearance:
Yellow powder.
CAS:
491-70-3
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
InChiKey:
IQPNAANSBPBGFQ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 491-70-3. Formula: C15H10O6. MW: 286.24. Luteolin is a hydroxylated flavone derivative that is a strong antioxidant and radical scavenger. It has multiple activities, such as anticancer, antimetastatic, anti-adipogenic, anti-inflammatory and neuroprotective. Luteolin has been described as a fatty acid synthase (FASN), 15-lipoxygenase, alpha-glucosidase, PPAR-gamma, topoisomerase I, PI3K/Akt, RSK1/RSK2 and HDAC inhibitor.
MDL:
MFCD00017309
Molecular Formula:
C15H10O6
Molecular Weight:
286.24
Package Type:
Vial
Product Description:
Luteolin is a hydroxylated flavone derivative that is a strong antioxidant and radical scavenger. It has multiple activities, such as anticancer, antimetastatic, anti-adipogenic, anti-inflammatory and neuroprotective. Luteolin has been described as a fatty acid synthase (FASN), 15-lipoxygenase, alpha-glucosidase, PPAR-gamma, topoisomerase I, PI3K/Akt, RSK1/RSK2 and HDAC inhibitor.
Purity:
>98% (TLC)
SMILES:
OC1=C2C(OC(C3=CC(O)=C(O)C=C3)=CC2=O)=CC(O)=C1
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF (20mg/ml), methanol or ethanol (5mg/ml). Slightly soluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) Y.T. Huang, et al.; Br. J. Pharmacol. 128, 999 (1999) | (2) J.S. Kim, et al.; Biosci. Biotechnol. Biochem. 64, 2458 (2000) | (3) H. Ueda, et al.; Biol. Pharm. Bull. 25, 1197 (2002) | (4) A.R. Chowdhury, et al.; Biochem. J. 366, 653 (2002) | (5) L. Sartor, et al.; Biochem. Pharmacol. 64, 229 (2002) | (6) C.D. Sadik, et al.; Biochem. Pharmacol. 65, 773 (2003) | (7) D.T. Coleman, et al.; Mol. Cancer Ther. 8, 214 (2009) | (8) K. Xu, et al.; Molecules 14, 3486 (2009) | (9) H.S. Park, et al.; Biofactors 35, 373 (2009) | (10) S. Attoub, et al.; Eur. J. Pharmacol. 651, 18 (2011) | (11) H.Y. Kim, et al.; Phytother. Res. 27, 1481 (2013) | (12) K.M. Reipas, et al.; Oncotarget 4, 329 (2013)