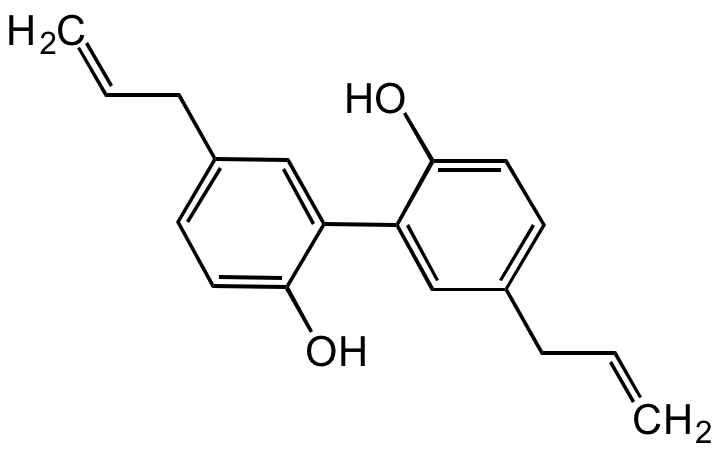
Magnolol
| Code | Size | Price |
|---|
| CDX-M0170-M010 | 10 mg | £72.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
5,5'-Diallyl-2,2'-biphenyldiol; NSC 293099
Appearance:
White to off-white powder.
CAS:
528-43-8
Class:
9
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS05,GHS07,GHS09
Handling Advice:
Protect from light and moisture.
Hazards:
H315, H318, H335, H411
InChi:
InChI=1S/C18H18O2/c1-3-5-13-7-9-17(19)15(11-13)16-12-14(6-4-2)8-10-18(16)20/h3-4,7-12,19-20H,1-2,5-6H2
InChiKey:
VVOAZFWZEDHOOU-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 528-43-8. Formula: C18H18O2. MW: 266.33. Magnolol is a bioactive compound isolated from the bark of M. officinalis that has been used in Asian traditional medicine for the treatment of anxiety, sleep disorders, and allergic diseases. Magnolol is an antifungal, antibacterial and antioxidant compound. It demonstrates anti-inflammatory activity by interferring with NF-kappaB signalling and NLRP3 inhibition. Magnolol can activate cannabinoid (CB) receptors, behaving as a partial agonist with selectivity for the peripheral CB2 subtype versus central CB1. It been identified as modulators of the GABAA receptors in vitro. It is a potent antitumor and antiangiogenic compound. Shown to inhibit HIF-1alpha/VEGF signalling, inhibiting invasion and metastasis through MMP2/MMP9 and inhibiting microtubule polymerization. Induces apoptosis through EGFR/PI3K/Akt pathway. Shows anti-diabetic/anti-obesity activity targeting nuclear receptors retinoic X receptor alpha (RXRalpha) and peroxisome proliferator activated receptor gamma (PPARgamma). In addition promotes thermogenesis.
MDL:
MFCD00016658
Molecular Formula:
C18H18O2
Molecular Weight:
266.33
Package Type:
Vial
PG:
III
Precautions:
P261, P273, P280, P305+P351+P338
Product Description:
Magnolol is a bioactive compound isolated from the bark of M. officinalis that has been used in Asian traditional medicine for the treatment of anxiety, sleep disorders, and allergic diseases. Magnolol is an antifungal, antibacterial and antioxidant compound. It demonstrates anti-inflammatory activity by interferring with NF-kappaB signalling and NLRP3 inhibition. Magnolol can activate cannabinoid (CB) receptors, behaving as a partial agonist with selectivity for the peripheral CB2 subtype versus central CB1. It been identified as modulators of the GABAA receptors in vitro. It is a potent antitumor and antiangiogenic compound. Shown to inhibit HIF-1alpha/VEGF signalling, inhibiting invasion and metastasis through MMP2/MMP9 and inhibiting microtubule polymerization. Induces apoptosis through EGFR/PI3K/Akt pathway. Shows anti-diabetic/anti-obesity activity targeting nuclear receptors retinoic X receptor alpha (RXRalpha) and peroxisome proliferator activated receptor gamma (PPARgamma). In addition promotes thermogenesis.
Purity:
>98% (HPLC)
Signal Word:
Danger
SMILES:
OC1=CC=C(CC=C)C=C1C2=CC(CC=C)=CC=C2O
Solubility Chemicals:
Soluble in ethanol (1mg/ml), methanol (1mg/ml), DMSO (10mg/ml) or DMF (10mg/ml). Insoluble in water.
Transportation:
Excepted Quantity
UN Nummer:
3077
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) K. Watanabe, et al.; Planta Med. 49, 103 (1983) | (2) J.P. Wang, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 346, 707 (1992) | (3) Y. Maruyama, et al.; J. Nat. Prod. 61, 135 (1998) | (4) K.H. Bang, et al.; Arch. Pharm. Res. 23, 46 (2000) | (5) K.Y. Ho, et al.; Phytother. Res. 15, 139 (2001) | (6) J. Ai, et al.; Pharmacology 63, 34 (2001) | (7) K. Ikeda & H. Nagase; Biol. Pharm. Bull. 25, 1546 (2002) | (8) K. Ikeda, et al.; Phytother. Res. 17, 933 (2003) | (9) J. Park, et al.; Eur. J. Pharmacol. 496, 189 (2004) | (10) A.K. Tse, et al.; Mol. Immunol. 44, 2647 (2007) | (11) D.H. Lee, et al.; J. Cell Biochem. 106, 1113 (2009) | (12) E.S. Hwang & K.K. Park; Biosci. Biotechnol. Biochem. 74, 961 (2010) | (13) H. Zhang, et al.; PLoS One 6, e28253 (2011) | (14) V. Rempel, et al.; ACS Med. Chem. Lett. 4, 41 (2013) | (15) M.C. Chen, et al.; Biochem. Pharmacol. 85, 1278 (2013) | (16) Y. Sakaue, et al.; Microbiol. Immunol. 60, 10 (2016) | (17) J. Shen, et al.; Cell Physiol. Biochem. 42, 1789 (2017) | (18) F. Huang, et al.; Mol. Med. Rep. 16, 4817 (2017) | (19) H.A. Parray, et al.; Nutrition 50, 82 (2018)