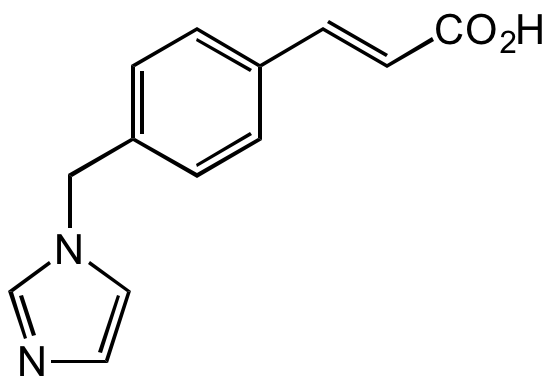
Ozagrel
| Code | Size | Price |
|---|
| CDX-O0141-M050 | 50 mg | £218.00 |
Quantity:
| CDX-O0141-M100 | 100 mg | £389.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
OKY-046; (E)-3-(4-((1H-Imidazol-1-yl)methyl)phenyl)acrylic acid
Appearance:
White to off-white crystalline powder.
CAS:
82571-53-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302
InChi:
InChI=1S/C13H12N2O2/c16-13(17)6-5-11-1-3-12(4-2-11)9-15-8-7-14-10-15/h1-8,10H,9H2,(H,16,17)/b6-5+
InChiKey:
SHZKQBHERIJWAO-AATRIKPKSA-N
Long Description:
Chemical. CAS: 82571-53-7. Formula: C13H12N2O2. MW: 228.2. Synthetic. Ozagrel, an antithrombic drug, is a potent and selective TXA Synthase (thromboxane A2 ; TXAS) inhibitor. Acts as a selective inhibitor of TXAS with an IC50=11nM. The beneficial effects of TXAS inhibition by ozagrel include improved motor coordination after experimental stroke, and antihypertensive effects in spontaneously hypertensive rats.
MDL:
MFCD00911569
Molecular Formula:
C13H12N2O2
Molecular Weight:
228.2
Package Type:
Vial
Precautions:
P280, P305+P351+P338
Product Description:
Ozagrel, an antithrombic drug, is a potent and selective TXA Synthase (thromboxane A2 ; TXAS) inhibitor. Acts as a selective inhibitor of TXAS with an IC50=11nM. The beneficial effects of TXAS inhibition by ozagrel include improved motor coordination after experimental stroke, and antihypertensive effects in spontaneously hypertensive rats.
Purity:
>98%
Signal word:
Warning
SMILES:
O=C(/C=C/C1=CC=C(CN2C=NC=C2)C=C1)O
Solubility Chemicals:
Soluble in DMSO (5mg/ml) or DMF (5mg/ml). Sligthly solube in ethanol (1mg/ml). Insoluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) J.B. Press, et al.; J. Med. Chem. 29, 816 (1986) | (2) K. Morita, et al.; J. Pharmacobiodyn. 11, 519 (1988)| (3) K. Kawakatsu, et al.; Int. J. Clin. Pharmacol. Ther. Toxicol. 28, 158 (1990)| (4) K. Ichikawa, et al.; Pharmacol. 59, 257 (1999) | (5) O. Moussa, et al.; Oncogene. 27, 55 (2008) | (6) Y. Ishitsuka, et al.; J. Pharmacol. Sci. 111, 211 (2009)