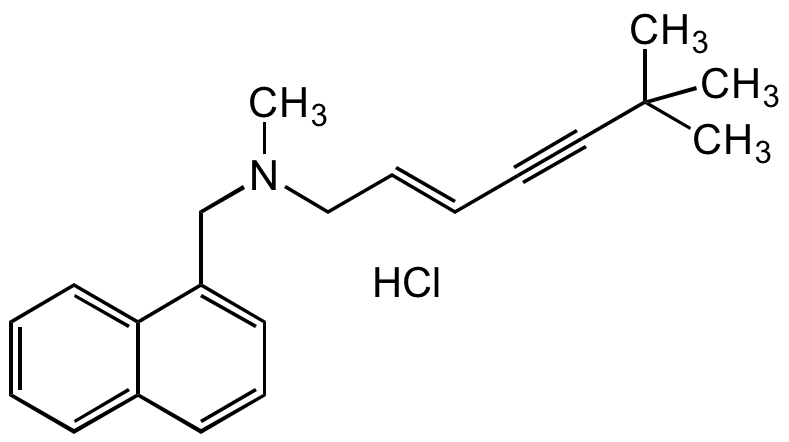
Terbinafine hydrochloride
| Code | Size | Price |
|---|
| CDX-T0207-G001 | 1 g | £145.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
trans-N-(6,6-Dimethyl-2-hepten-4-ylyl)-N-methyl-1-naphthylmethylamine hydrochloride; N-[(2E)-6,6-dimethyl-2-hepten-4-yn-1-yl]-N-methyl-1-naphthalenemethanamine hydrochloride
Appearance:
White to off-white powder.
CAS:
78628-80-5
Class:
9
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07,GHS09
Hazards:
H315, H319, H335, H410
InChi:
InChI=1S/C21H25N.ClH/c1-21(2,3)15-8-5-9-16-22(4)17-19-13-10-12-18-11-6-7-14-20(18)19;/h5-7,9-14H,16-17H2,1-4H3;1H/b9-5+;
InChiKey:
BWMISRWJRUSYEX-SZKNIZGXSA-N
Long Description:
Chemical. CAS: 78628-80-5. Formula: C21H25N . HCl. MW: 291.4 . 36.5. Synthetic. Terbinafine is an antifungal and antimycotic compound that is highly active against dermatophytes, mold, other basic fungi, and some strains of yeast. It is clinically used to treat nail and skin infections. Inhibits ergosterol synthesis, essential component of fungal cell membranes. Potent non-competitive inhibitor at the stage of squalene epoxidation (IC50=30nM for C. albicans). Selective activator of the K2P channel TASK3 (pEC50 = 6.2). Exhibits >10-fold selectivity for TASK3 over TREK2, TRESK, THIK1 and TASK2. Also inhibits TWIK1 (pIC50 = 5.69). K2P channels might also be a target for ist antifungal activity. Shown to exhibit at higher concentrations anti-tumor and anti-angiogenic activity by inducing cell cycle arrest, and to display interesting anti-inflammatory and free radical scavenging activities.
MDL:
MFCD00145430
Molecular Formula:
C21H25N . HCl
Molecular Weight:
291.4 . 36.5
Package Type:
Vial
PG:
III
Precautions:
P261, P273, P305+P351+P338, P501
Product Description:
Terbinafine is an antifungal and antimycotic compound that is highly active against dermatophytes, mold, other basic fungi, and some strains of yeast. It is clinically used to treat nail and skin infections. Inhibits ergosterol synthesis, essential component of fungal cell membranes. Potent non-competitive inhibitor at the stage of squalene epoxidation (IC50=30nM for C. albicans). Selective activator of the K2P channel TASK3 (pEC50 = 6.2). Exhibits >10-fold selectivity for TASK3 over TREK2, TRESK, THIK1 and TASK2. Also inhibits TWIK1 (pIC50 = 5.69). K2P channels might also be a target for ist antifungal activity. Shown to exhibit at higher concentrations anti-tumor and anti-angiogenic activity by inducing cell cycle arrest, and to display interesting anti-inflammatory and free radical scavenging activities.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
CN(C/C=C/C#CC(C)(C)C)CC1=CC=CC2=CC=CC=C21.Cl
Solubility Chemicals:
Soluble in ethanol (30mg/ml), DMSO (10mg/ml), DMF (10mg/ml), methanol (50 mg/ml). Sparingly soluble in acetone or water.
Source / Host:
Synthetic
Transportation:
Excepted Quantity
UN Nummer:
3077
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) G. Petranyi, et al.; Science 224, 1239 (1984) | (2) N.S. Ryder & M.C. Dupont; Biochem. J. 230, 765 (1985) | (3) G. Petranyi, et al.; Antimicrob. Agents Chemother. 31, 1365 (1987) | (4) N. S. Ryder, et al.; Br. J. Dermatol. 126, 39 (1992) | (5) T. Rosen, et al.; Int. J. Dermatol. 36, 788 (1997) | (6) B. Favre, et al.; Arch. Biochem. Biophys. 340, 265 (1997) | (7) C.S. Sander, et al.; Mycoses 45, 152 (2002) | (8) W.S. Lee, et al.; Int. J. Canc. 106, 125 (2003) | (9) P.Y. Ho, et al.; J. Canc. 111, 51 (2004) | (10) P.D. Wright, et al.; BBRC 493, 444 (2017)