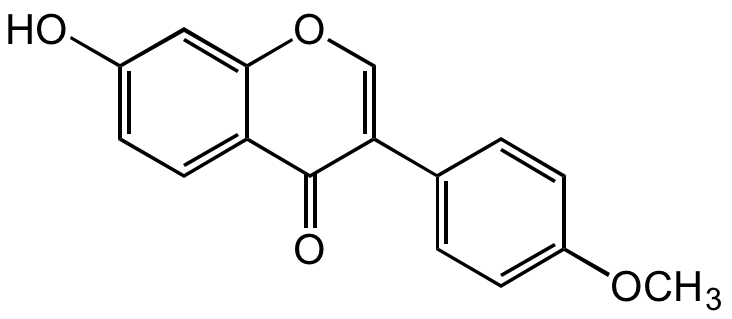
Formononetin
| Code | Size | Price |
|---|
| CDX-F0339-M050 | 50 mg | £44.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Biochanin B; NSC 93360; 7-Hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one; 7-Hydroxy-3-(4-methoxyphenyl)chromone, 7-Hydroxy-4'-methoxyisoflavone
Appearance:
White to off-white powder.
CAS:
485-72-3
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C16H12O4/c1-19-12-5-2-10(3-6-12)14-9-20-15-8-11(17)4-7-13(15)16(14)18/h2-9,17H,1H3
InChiKey:
HKQYGTCOTHHOMP-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 485-72-3. Formula: C16H12O4. MW: 268.26. Formononetin is an isoflavonoid phytoestrogenic compound found in soy-based foods and is the precursor of daidzein. It acts as an agonist of the aryl hydrocarbon receptor and a selective inhibitor of ADH gamma (the gamma-isoform of alcohol dehydrogenase). It displays potent antitumor, antiviral, anti-inflammatory (inhibits NLRP3 inflammasome), antioxidant, neuroprotective and neuroinflammatory properties. Also shown to have vasorelaxant, antihypertensive and antihyperglycemic (AMPK activator) properties. Induces cell cycle arrest and apoptosis in several cancer cells in vitro and in vivo and shows anti-angiogenic (FGFR2 inhibitor), anti-invasive and anti-migratory effects. Increases adipocyte thermogenesis by modulating PPARgamma activity. Shown to promote early fracture healing through stimulating angiogenesis by up-regulating VEGFR-2/Flk-1 and endothelial repair and wound healing in a process involving the over-expression of Egr-1 transcription factor through the regulation of the ERK1/2 and p38 MAPK pathways.
MDL:
MFCD00016948
Molecular Formula:
C16H12O4
Molecular Weight:
268.26
Package Type:
Vial
Product Description:
Formononetin is an isoflavonoid phytoestrogenic compound found in soy-based foods and is the precursor of daidzein. It acts as an agonist of the aryl hydrocarbon receptor and a selective inhibitor of ADH gamma (the gamma-isoform of alcohol dehydrogenase). It displays potent antitumor, antiviral, anti-inflammatory (inhibits NLRP3 inflammasome), antioxidant, neuroprotective and neuroinflammatory properties. Also shown to have vasorelaxant, antihypertensive and antihyperglycemic (AMPK activator) properties. Induces cell cycle arrest and apoptosis in several cancer cells in vitro and in vivo and shows anti-angiogenic (FGFR2 inhibitor), anti-invasive and anti-migratory effects. Increases adipocyte thermogenesis by modulating PPARgamma activity. Shown to promote early fracture healing through stimulating angiogenesis by up-regulating VEGFR-2/Flk-1 and endothelial repair and wound healing in a process involving the over-expression of Egr-1 transcription factor through the regulation of the ERK1/2 and p38 MAPK pathways. Binding to human cell-surface receptor HSPA5 and prevents SARS-CoV-2 spike protein from binding to HSPA5 SBDbeta in silico.
Purity:
>98% (HPLC)
SMILES:
OC1=CC=C(C(C(C2=CC=C(OC)C=C2)=CO3)=O)C3=C1
Solubility Chemicals:
Soluble in DMSO (30mg/ml), DMF (30mg/ml) or ethanol (1mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) W.M. Keung; BBRC 215, 1137 (1995) | (2) S. Toda and Y. Shirataki; Phytother. Res. 13, 163 (1999) | (3) C.K. Wong & W.M. Keung; J. Steroid Biochem. Mol. Biol. 71, 191 (1999) | (4) B.L. Pool-Zobel, et al.; Carcinogenesis 21, 1247 (2000) | (5) S. Medjakovic & A. Jungbauer ; J. Steroid Biochem. Mol. Biol 108, 171 (2008) | (6) H. Mu, et al.; Phytomedicine 16, 314 (2009) | (7) J.E . Huh, et al.; Int. Immunopharmacol. 9, 1357 (2009) | (8) J.E . Huh, et al.; Int. Immunopharmacol. 11, 46 (2011) | (9) T. Sun, et al.; Acta Pharmacol. Sin. 32, 1009 (2011) | (10) J. Chen, et al.; Horm. Metab. Res. 43, 681 (2011) | (11) M. Sun, et al.; J. Alzheimers Dis. 28, 795 (2012) | (12) Y. Ye, et al.; Horm. Metab. Res. 44, 263 (2012) | (13) K.K. Auyeung, et al.; Oncol. Rep. 28, 2188 (2012) | (14) R. Zhou, et al.; Horm. Metab. Res. 46, 753 (2014) | (15) Y. Yang, et al.; Int. J. Clin. Exp. Pathol. 7, 8453 (2014) | (16) X.Y. Wu, et al.; Oncotarget 6, 44563 (2015) | (17) H. Wang, et al.; Virol. J. 12, 1 (2015) | (18) G. Qiu, et al.; Exp. Biol. Med. 242, 223 (2017) | (19) J. Gautam, et al.; Br. J. Nutr. 117, 645 (2017) | (20) T. Nie, et al.; Br. J. Pharmacol. 175, 1439 (2018) | (21) D. Wu, et al.; Mediators Inflamm. 2018, 3048532 (2018) | (22) A. El-Bakoush & O.A. Olajide; Int. Immunopharmacol. 61, 325 (2018) | Natural products may interfere with SARS-CoV-2 attachment to the host cell: A.A. Elfiky, J. Biomol. Struct. Dyn. 1 (2020)