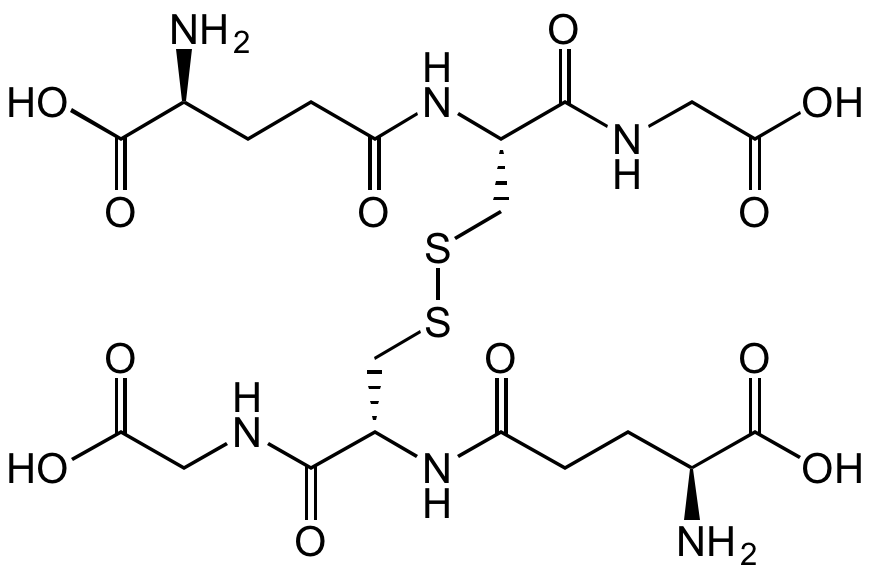
L-Glutathione oxidized
| Code | Size | Price |
|---|
| CDX-G0039-G001 | 1 g | £59.00 |
Quantity:
| CDX-G0039-G005 | 5 g | £126.00 |
Quantity:
| CDX-G0039-G010 | 10 g | £237.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
GSSG; Glutathiol; Oxiglutatione; Glutathone disulfide; Glutathione-S-S-glutathione; Glutathione-SSG; (gamma-Glu-Cys-Gly)2; N,N?-[Dithiobis[1-[(carboxymethyl)carbamoyl]ethylene]]diglutamine
Appearance:
White powder.
CAS:
27025-41-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C20H32N6O12S2/c21-9(19(35)36)1-3-13(27)25-11(17(33)23-5-15(29)30)7-39-40-8-12(18(34)24-6-16(31)32)26-14(28)4-2-10(22)20(37)38/h9-12H,1-8,21-22H2,(H,23,33)(H,24,34)(H,25,27)(H,26,28)(H,29,30)(H,31,32)(H,35,36)(H,37,38)/t9-,10-,11-,12-/m0/s1
InChiKey:
YPZRWBKMTBYPTK-BJDJZHNGSA-N
Long Description:
Chemical. CAS: 27025-41-8. Formula: C20H32N6O12S2. MW: 612.63. Glutathione can occur in reduced (GSH), oxidized (GSSG), or in mixed disulfide forms and is ubiquitous in multiple biological systems serving as the major thiol-disulfide redox buffer of the cell. Glutathione, Oxidized (GSSG) is the oxidized version of the naturally occurring and very important antioxidant glutathione (GSH). In vivo GSSG is reduced back to GSH via the NADPH-dependent enzyme glutathione reductase. The ratio of GSH to GSSG is often used to gauge the level of oxidative stress in cells, with higher concentrations of GSSG implying more oxidative stress, therefore beeing an important bioindicator of cellular health. GSSG functions as a hydrogen acceptor in the enzymatic determination of NADP+ and NADPH and can be a proximal donor in S-glutathionylation post translational modifications. GSSG, along with glutathione and S-nitrosoglutathione (GSNO), have been found to bind to the glutamate recognition site of the NMDA and AMPA receptors (via their gamma-glutamyl moieties), and may be endogenous neuromodulators. GSSG can be used as substrate for enzymatically assaying glutathione reductase.
MDL:
MFCD00063106
Molecular Formula:
C20H32N6O12S2
Molecular Weight:
612.63
Package Type:
Vial
Product Description:
Glutathione can occur in reduced (GSH), oxidized (GSSG), or in mixed disulfide forms and is ubiquitous in multiple biological systems serving as the major thiol-disulfide redox buffer of the cell. Glutathione, Oxidized (GSSG) is the oxidized version of the naturally occurring and very important antioxidant glutathione (GSH). In vivo GSSG is reduced back to GSH via the NADPH-dependent enzyme glutathione reductase. The ratio of GSH to GSSG is often used to gauge the level of oxidative stress in cells, with higher concentrations of GSSG implying more oxidative stress, therefore beeing an important bioindicator of cellular health. GSSG functions as a hydrogen acceptor in the enzymatic determination of NADP+ and NADPH and can be a proximal donor in S-glutathionylation post translational modifications. GSSG, along with glutathione and S-nitrosoglutathione (GSNO), have been found to bind to the glutamate recognition site of the NMDA and AMPA receptors (via their gamma-glutamyl moieties), and may be endogenous neuromodulators. GSSG can be used as substrate for enzymatically assaying glutathione reductase.
Purity:
>98% (HPLC)
SMILES:
OC([C@@H](N)CCC(N[C@@H](CSSC[C@@H](C(NCC(O)=O)=O)NC(CC[C@H](N)C(O)=O)=O)C(NCC(O)=O)=O)=O)=O
Solubility Chemicals:
Soluble in water, DMSO or ethanol.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) A. Meister; J. Biol. Chem. 263, 17205 (1988) | (2) S.M. Deneke & B.L. Fanburg; Am. J. Physiol. 257, L163 (1989) | (3) V. Varga, et al.; Neurochem. Res. 22, 1165 (1997) | (4) A. Pompella, et al.; Biochem. Pharmacol. 66, 1499 (2003) | (5) D. Giustarini, et al.; J. Cell. Mol. Med. 8, 201 (2004) | (6) P. Ghezzi; Free Radic. Res. 39, 573 (2005) | (7) P. Steullet, et al.; Neuroscience. 137, 807 (2006) | (8) J.B. Owen & D.A. Butterfield; Methods Mol. Biol. 648, 269 (2010)