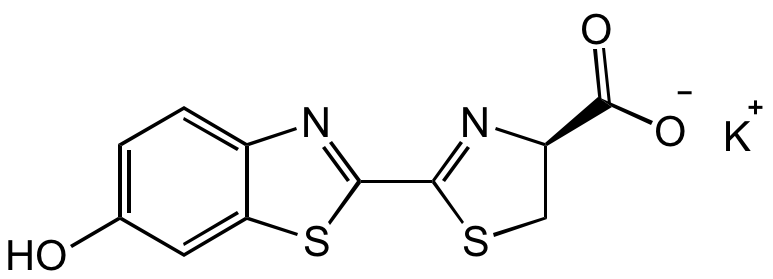
D-Luciferin potassium salt
| Code | Size | Price |
|---|
| CDX-L0009-M050 | 50 mg | £48.00 |
Quantity:
| CDX-L0009-M250 | 250 mg | £126.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(S)-2-(6-Hydroxy-2-benzothiazolyl)-2-thiazoline-4-carboxylic acid potassium salt; 4,5-Dihydro-2-(6-hydroxy-2-benzothiazolyl)-4-thiazolecarboxylic acid potassium salt; Firefly luciferin potassium salt
Appearance:
Light yellow solid.
CAS:
115144-35-9
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C11H8N2O3S2.K/c14-5-1-2-6-8(3-5)18-10(12-6)9-13-7(4-17-9)11(15)16;/h1-3,7,14H,4H2,(H,15,16);/q;+1/p-1/t7-;/m1./s1
InChiKey:
UMBKGTQQGYPQBE-OGFXRTJISA-M
Long Description:
Chemical. CAS: 115144-35-9. Formula: C11H7KN2O3S2. MW: 318.41. D-Luciferin is the most popular and versatile bioluminescent substrate. Firefly luciferase produces light by the ATP-dependent oxidation (Mg2+ as cofactor) of luciferin. It emits a characteristic yellow-green emission in the presence of oxygen, which shifts to red light in vivo. The 560nm chemiluminescence from this reaction peaks within seconds, with light output that is proportional to luciferase activity when luciferin and ATP are present in excess. Firefly luciferase has long been conjugated to antibodies and used as a label in immunoassays using luciferin as the substrate for detection. One particular advantage to the enzyme is that there is low endogenous luciferase activity in mammalian tissues besides its high sensitivity. Through the utilization of ATP, the reaction can be further used to indicate the presence of energy or life in order to function as a life-death stain. Luciferin is a common reagent used throughout the biotechnology field and specifically for in vivo imaging. Luciferase labeled tumor cells, stem cells or infectious diseases are often inoculated into research animals such as rats or mice for investigation. The injection of luciferin allows for the real-time, non-invasive monitoring of disease progression and/or drug efficacy in these model systems through Bioluminescence Imaging (BLI). Luciferin is also commonly used for in vitro research, including luciferase and ATP assays, gene reporter assays, high throughput sequencing and various contamination assays (e.g. determine cell viability and bacteria counting).
MDL:
MFCD00044928
Molecular Formula:
C11H7KN2O3S2
Molecular Weight:
318.41
Package Type:
Vial
Product Description:
D-Luciferin is the most popular and versatile bioluminescent substrate. Firefly luciferase produces light by the ATP-dependent oxidation (Mg2+ as cofactor) of luciferin. It emits a characteristic yellow-green emission in the presence of oxygen, which shifts to red light in vivo. The 560nm chemiluminescence from this reaction peaks within seconds, with light output that is proportional to luciferase activity when luciferin and ATP are present in excess. Firefly luciferase has long been conjugated to antibodies and used as a label in immunoassays using luciferin as the substrate for detection. One particular advantage to the enzyme is that there is low endogenous luciferase activity in mammalian tissues besides its high sensitivity. Through the utilization of ATP, the reaction can be further used to indicate the presence of energy or life in order to function as a life-death stain. Luciferin is a common reagent used throughout the biotechnology field and specifically for in vivo imaging. Luciferase labeled tumor cells, stem cells or infectious diseases are often inoculated into research animals such as rats or mice for investigation. The injection of luciferin allows for the real-time, non-invasive monitoring of disease progression and/or drug efficacy in these model systems through Bioluminescence Imaging (BLI). Luciferin is also commonly used for in vitro research, including luciferase and ATP assays, gene reporter assays, high throughput sequencing and various contamination assays (e.g. determine cell viability and bacteria counting).
Purity:
>95% (HPLC)
SMILES:
OC1=CC=C2C(SC(C3=N[C@@H](C([O-])=O)CS3)=N2)=C1.[K+]
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or water (10mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) V.T. Nguyen, et al.; Anal. Biochem. 171, 404 (1988) | (2) S.P. Crouch, et al.; J. Immunol. Methods 160, 81 (1993) | (3) N. Lembert; Biochem. J. 317, 273 (1996) | (4) S.R. Ford, et al.; Methods Mol. Biol. 102, 3 (1998) | (5) J.D. Day, et al.; Luminescence 19, 8 (2004) (Review) | (6) V.R. Viviani & Y. Ohmiya; Luminescence 21, 262 (2006) | (7) M.L. Schipper, et al.; Mol. Imaging Biol. 8, 218 (2006) | (8) S. Palomba, et al.; Langmuir 22, 5451 (2006) | (9) S.T. Adams & S.C. Miller; Curr. Opin. Chem. Biol. 21, 112 (2014) (Review) | (10) T. Ishimoto, et al.; J. Biol. Pharm. Bull. 2015, 1969 (2015) | (11) M. Kiyama, et al.; Curr. Top. Med. Chem. 16, 2648 (2016) (Review)