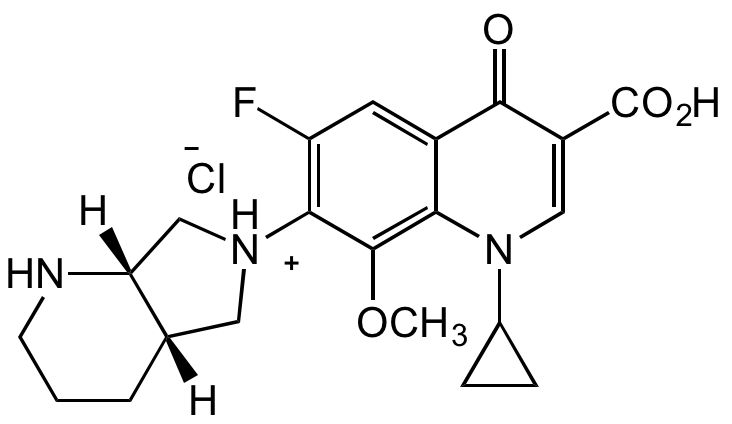
Moxifloxacin hydrochloride
| Code | Size | Price |
|---|
| CDX-M0189-M050 | 50 mg | £89.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
BAY 12-8039; Alelox; 1-Cyclopropyl-6-fluoro-8-methoxy-7-[(4aS,7aS)-octahydropyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride
Appearance:
Light yellow powder.
CAS:
186826-86-8
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C21H24FN3O4.ClH/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24;/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28);1H/t11-,16+;/m0./s1
InChiKey:
IDIIJJHBXUESQI-DFIJPDEKSA-N
Long Description:
Chemical. CAS: 186826-86-8. Formula: C21H24FN3O4 . HCl. MW: 401.43 . 36.46. Moxifloxacin is a fourth-generation synthetic broad-spectrum fluoroquinolone antibiotic. It is used against both Gram-positive and Gram-negative bacteria and to treat bacterial infections associated with bronchitis, sinusitis, and other conditions. Fluoroquinolones stabilize DNA strand breaks created by DNA gyrase (Topo II) and topoisomerase IV by binding to the enzyme-DNA complex. Compared to mammalian DNA gyrase, moxifloxacin has 100 times higher affinity for bacterial DNA gyrase. Can also be used as reference compound.
MDL:
MFCD00949117
Molecular Formula:
C21H24FN3O4 . HCl
Molecular Weight:
401.43 . 36.46
Package Type:
Vial
Product Description:
Moxifloxacin is a fourth-generation synthetic broad-spectrum fluoroquinolone antibiotic. It is used against both Gram-positive and Gram-negative bacteria and to treat bacterial infections associated with bronchitis, sinusitis, and other conditions. Fluoroquinolones stabilize DNA strand breaks created by DNA gyrase (Topo II) and topoisomerase IV by binding to the enzyme-DNA complex. Compared to mammalian DNA gyrase, moxifloxacin has 100 times higher affinity for bacterial DNA gyrase. Can also be used as reference compound.
Purity:
>97% (NMR)
SMILES:
O=C1C(C(O)=O)=CN(C2CC2)C3=C(OC)C([NH+]4C[C@](NCCC5)([H])[C@]5([H])C4)=C(F)C=C31.[Cl-]
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or water (5mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) M. Souli, et al.; Int. J. Antimicrob. Agents. 10, 23 (1998) | (2) C. Ballow, et al.; Clin. Ther. 21, 513 (1999) | (3) A. Dalhoff, et al.; Clin. Infect. Dis. 32, S22 (2001) | (4) R. Kishii, et al.; Antimicrob. Agents Chemother. 47, 77 (2003) | (5) M. Miravitlles; J. Chron. Obstruct. Pulmon. Dis. 2, 191 (2007) | (6) M. Miravitlles & A. Anzueto; Expert Opin. Pharmacother. 9, 1755 (2008) (Review) | (7) H. Takiff & E. Guerrero; Antimicrob. Agents Chemother. 55, 5421 (2011) | (8) M.M. Al Omari, et al.; Profiles Drug Subst. Excip. Relat. Methodol. 39, 299 (2014) (Review)