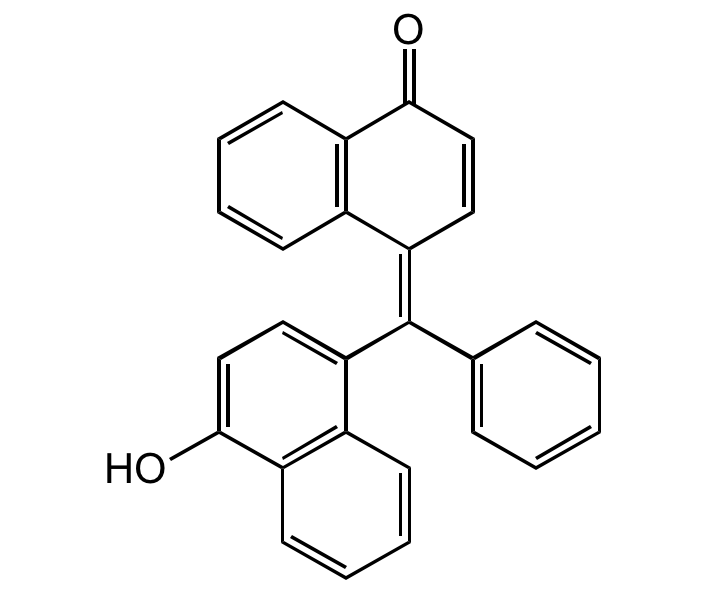
alpha-Naphtholbenzein
| Code | Size | Price |
|---|
| CDX-N0116-G010 | 10 g | £84.00 |
Quantity:
| CDX-N0116-G050 | 50 g | £230.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
4,4'-(alpha-Hydroxybenzylidene)di-1-naphthol; p-Naphtholbenzein; NSC 9862
Appearance:
Dark red powder.
CAS:
145-50-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C27H18O2/c28-25-16-14-23(19-10-4-6-12-21(19)25)27(18-8-2-1-3-9-18)24-15-17-26(29)22-13-7-5-11-20(22)24/h1-17,28H/b27-24-
InChiKey:
VDDWRTZCUJCDJM-PNHLSOANSA-N
Long Description:
Chemical. CAS: 145-50-6. Formula: C27H18O2. MW: 374.43. alpha-Naphtholbenzein is a dye generally used as an acid-base indicated. The pH-indicator has a visual transition from yellow (pH0-8.2) to turquoise (pH10). It is often used during sequential injection analysis technique done for acid-base titrations. It also has been used for food storage, determining bacterial growth in packed food, detecting viable cells and detecting enzymes.
MDL:
MFCD00078492
Molecular Formula:
C27H18O2
Molecular Weight:
374.43
Package Type:
Vial
Product Description:
alpha-Naphtholbenzein is a dye generally used as an acid-base indicated. The pH-indicator has a visual transition from yellow (pH0-8.2) to turquoise (pH10). It is often used during sequential injection analysis technique done for acid-base titrations. It also has been used for food storage, determining bacterial growth in packed food, detecting viable cells and detecting enzymes.
SMILES:
OC1=CC=C(/C(C2=CC=CC=C2)=C3C=CC(C4=C/3C=CC=C4)=O)C5=C1C=CC=C5
Solubility Chemicals:
Soluble in ethanol or methanol. Insoluble in water.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) T. Higuchi, et al.; Anal. Chem. 28, 1120 (1956) | (2) M. Silla, et al.; Talanta 52.1, 91 (2000)