Neutral Red 5
| Code | Size | Price |
|---|
| CDX-N0249-G025 | 25 g | £72.00 |
Quantity:
| CDX-N0249-G100 | 100 g | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
C.I. 50040; 3-Amino-7-dimethylamino-2-methylphenazine hydrochloride; Basic Red 5; Toluylene red
Appearance:
Red to dark red powder.
CAS:
553-24-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C15H16N4.ClH/c1-9-6-13-15(8-11(9)16)18-14-7-10(19(2)3)4-5-12(14)17-13;/h4-8H,1-3H3,(H2,16,18);1H
InChiKey:
GIBXNAFNURIQII-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 553-24-2. Formula: C15H17CIN4. MW: 288.78. Neutral red acts as a cellular pH indicator, changing from red to yellow between pH 6.8 and 8.0. More important, it is a dye used for staining in histology. It stains lysosomes red and is used as a general stain in histology, as a counterstain in combination with other dyes, and for many staining methods. It is applied in histology for nuclear staining, fluorescence staining, polychrome staining or for counter staining in Gram staining. It has also been incorporated in investigation of viruses, as a pH indicator, determination of DNA with optical and electrochemical methods, evalutaion of synthetic and naturally derived biomaterials. In microbiology, it is used in the MacConkey agar to differentiate bacteria for lactose fermentation. Neutral red is often used as a vital stain. Live cells incorporate neutral red into their lysosomes. As cells begin to die, their ability to incorporate neutral red diminishes. Thus, loss of neutral red uptake corresponds to loss of cell viability. The compound once taken up by cells can be used to acertain cell viability with a excitation maximum at 470nm and emission maximum at 580nm. Neutral red is added to some growth media for bacterial and cell cultures. Neutral red can be electrochemically polymerized and used as a redox mediator.
MDL:
MFCD00012651
Molecular Formula:
C15H17CIN4
Molecular Weight:
288.78
Package Type:
Vial
Product Description:
Neutral red acts as a cellular pH indicator, changing from red to yellow between pH 6.8 and 8.0. More important, it is a dye used for staining in histology. It stains lysosomes red and is used as a general stain in histology, as a counterstain in combination with other dyes, and for many staining methods. It is applied in histology for nuclear staining, fluorescence staining, polychrome staining or for counter staining in Gram staining. It has also been incorporated in investigation of viruses, as a pH indicator, determination of DNA with optical and electrochemical methods, evalutaion of synthetic and naturally derived biomaterials. In microbiology, it is used in the MacConkey agar to differentiate bacteria for lactose fermentation. Neutral red is often used as a vital stain. Live cells incorporate neutral red into their lysosomes. As cells begin to die, their ability to incorporate neutral red diminishes. Thus, loss of neutral red uptake corresponds to loss of cell viability. The compound once taken up by cells can be used to acertain cell viability with a excitation maximum at 470nm and emission maximum at 580nm. Neutral red is added to some growth media for bacterial and cell cultures. Neutral red can be electrochemically polymerized and used as a redox mediator.
Purity:
for microscopy
SMILES:
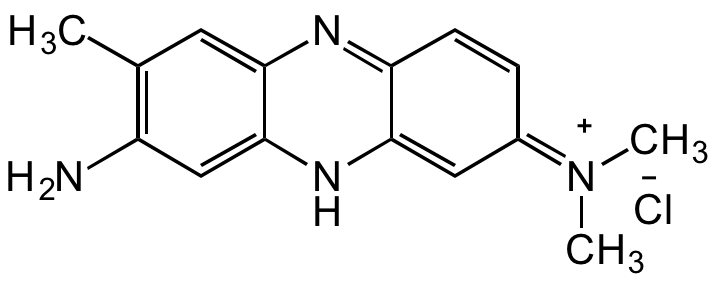
CC1=C(N)C=C(NC(C2=N3)=C/C(C=C2)=[N+](C)/C)C3=C1.[Cl-]
Solubility Chemicals:
Soluble in water (10mg/ml).
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Fluorescent Reagents
UNSPSC Number:
41105331
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) J. Winckler; Red. Prog. Histochem. Cytochem. 6, 1 (1974) | (2) P. Antal, et al.; Ann. Hematol. 70 259 (1995) | (3) V. Ricci, et al.; BBRC 292, 167 (2002) | (4) Y. Ni, et al.; Anal. Biochem. 352, 231 (2006) | (5) C.D. Link, et al.; J. Biol. Chem. 281, 1808 (2006) | (6) G. Repetto, et al.; Nat. Protoc. 3, 1125 (2008) | (7) M.A. Rauf, et al.; Chem. Cent. J. 2, 19 (2008) | (8) B. Chazotte; Cold Spring Harb. Protoc. 2011, pdb.prot5570 (2011) | (9) G. Ates, et al.; Methods Mol. Biol. 1601, 19 (2017) | (10) L. Xu, et al.; Chem. Commun. 52, 14330 (2016)