UBS109
| Code | Size | Price |
|---|
| AG-CR1-3704-M001 | 1 mg | £70.00 |
Quantity:
| AG-CR1-3704-5001 | 5 x 1 mg | £160.00 |
Quantity:
| AG-CR1-3704-M010 | 10 mg | £260.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Antibody Isotype: n/a
Antibody Clone: n/a
Regulatory Status: RUO
Shipping:
Blue Ice
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
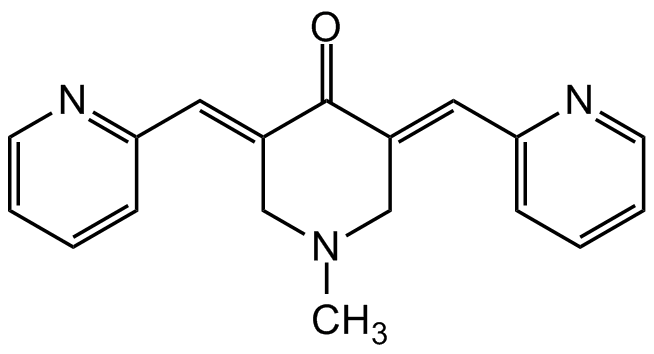
(3E,5E)-1-Methyl-3,5-bis(2-pyridinylmethylene)-4-piperidinone; Monocarbonyl Analog of Curcumin
Appearance:
Yellow solid.
CAS:
1258513-40-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.Protect from light.
Hazards:
H302, H315, H319, H335
InChi:
InChI=1S/C18H17N3O/c1-21-12-14(10-16-6-2-4-8-19-16)18(22)15(13-21)11-17-7-3-5-9-20-17/h2-11H,12-13H2,1H3/b14-10+,15-11+
InChiKey:
VCLPNFMJSSBBKX-WFYKWJGLSA-N
Long Description:
Chemical. CAS: 1258513-40-4. Formula: C18H17N3O. MW: 291.4. Most water soluble synthetic monocarbonyl analog of curcumin (MAC) for in vitro and in vivo application.
Potently inhibits NF-kappaB and its nuclear translocation by suppressing IKK-alpha and IKK-beta, consequently leading to apoptosis.
Potent DNA hypomethylating agent. Inhibits HSP90 and NF-kappaB leading to downregulation of DNA methyltransferase-1 (DNMT-1) expression.
Cytotoxic against several cancers in vitro and in vivo. Shows excellent activity against xenografts of head and neck squamous cell carcinoma, pancreatic cancer, colon cancer and breast cancer.
Antiangiogenic agent. Induces the downregulation of HIF-1alpha, HSP90, COX-2 and VEGF in tumor samples from xenograft models compared to untreated xenografts.
Shows preventive effects on bone loss induced by breast cancer cell bone metastasis. Found to have a potential stimulating effect on osteoblastogenesis and a suppressive effect on osteoclastogenesis in vitro through Smad activation and NF-kappaB inhibition. May have promise in the development into an antiosteoporotic agent capable of promoting new bone formation while simultaneously reducing bone resorption.
MDL:
MFCD28144029
Molecular Formula:
C18H17N3O
Molecular Weight:
291.4
Other data:
Note: Always prepare and use fresh solution. We recommend to use the fresh solution within the same day. The product can degrade in solution over time.
Package Type:
Vial
Precautions:
P261, P271, P280, P312, P302+P352, P304+P340, P305+P351+P338
Product Description:
Most water soluble synthetic monocarbonyl analog of curcumin (MAC) for in vitro and in vivo application. Potently inhibits NF-kappaB and its nuclear translocation by suppressing IKK-alpha and IKK-beta, consequently leading to apoptosis. Potent DNA hypomethylating agent. Inhibits HSP90 and NF-kappaB leading to downregulation of DNA methyltransferase-1 (DNMT-1) expression. Cytotoxic against several cancers in vitro and in vivo. Shows excellent activity against xenografts of head and neck squamous cell carcinoma, pancreatic cancer, colon cancer and breast cancer. Antiangiogenic agent. Induces the downregulation of HIF-1alpha, HSP90, COX-2 and VEGF in tumor samples from xenograft models compared to untreated xenografts. Shows preventive effects on bone loss induced by breast cancer cell bone metastasis. Found to have a potential stimulating effect on osteoblastogenesis and a suppressive effect on osteoclastogenesis in vitro through Smad activation and NF-kappaB inhibition. May have promise in the development into an antiosteoporotic agent capable of promoting new bone formation while simultaneously reducing bone resorption.
Purity:
>95% (HPLC)
Signal word:
Warning
SMILES:
O=C(/C(CN(C)C/1)=C/C2=NC=CC=C2)C1=CC3=CC=CC=N3
Solubility Chemicals:
Soluble in DMSO (10mg/ml). Insoluble in water.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-kB inhibition: M. Yamaguchi, et al.; Integr. Biol. 4, 905 (2012) | Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer: G. Nagaraju, et al.; Cancer Lett. 341, 195 (2013) | Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: involvement in osteoblastogenesis and osteoclastogenesis: M. Yamaguchi, et al.; Cell Tissue Res. 357, 245 (2014) | Liver S9 Fraction-Derived Metabolites of Curcumin Analogue UBS109: T.W. Moore, et al.; Med. Chem. Lett. 5, 288 (2014) | Curcumin analog UBS109 prevents bone marrow osteoblastogenesis and osteoclastogenesis disordered by coculture with breast cancer MDA-MB-231 bone metastatic cells in vitro: M. Yamaguchi, et al.; Mol. Cell Biochem. 401, 1 (2015) | Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs): D. Shetty, et al.; Molecules 20, 249 (2015) (Review) | Inhibition of breast cancer metastasis to the lungs with UBS109: M. Shoji, et al.; Oncotarget 9, 36102 (2018) | Curcumin and derivatives function through protein phosphatase 2A and presenilin orthologues in Dictyostelium discoideum: M. Cocorocchio, et al.; Dis. Mod. Mechan. 11, dmm032375 (2018)
Related Products
| Product Name | Product Code | Supplier | Curcumin (high purity) | AG-CN2-0059 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|