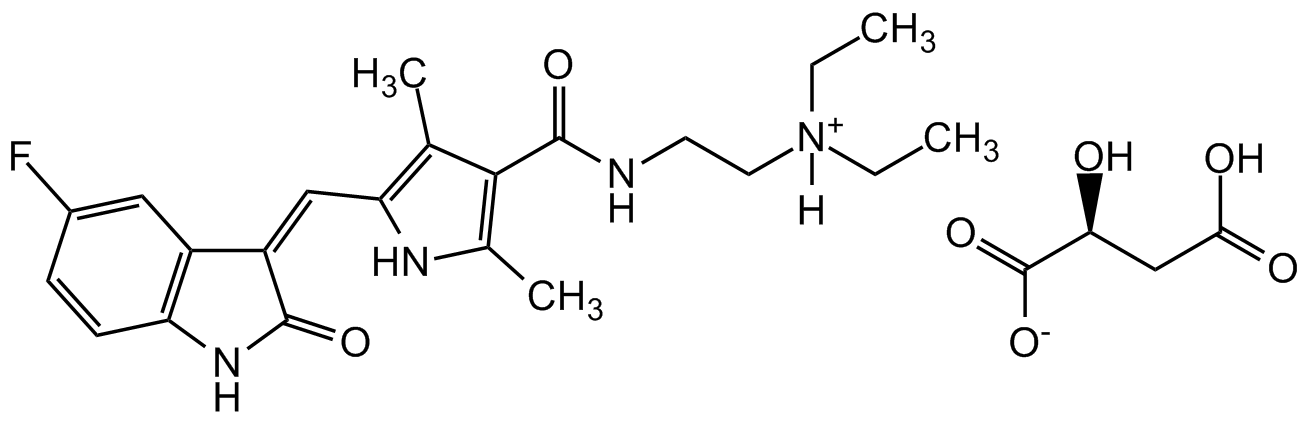
Sunitinib . malate
| Code | Size | Price |
|---|
| AG-CR1-3707-M001 | 1 mg | £40.00 |
Quantity:
| AG-CR1-3707-M005 | 5 mg | £65.00 |
Quantity:
| AG-CR1-3707-M025 | 25 mg | £85.00 |
Quantity:
| AG-CR1-3707-M500 | 500 mg | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
SU-11248
Appearance:
Solid.
CAS:
341031-54-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Hazards:
H360, H372
InChi:
InChI=1S/C22H27FN4O2.C4H6O5/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28;5-2(4(8)9)1-3(6)7/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28);2,5H,1H2,(H,6,7)(H,8,9)/b17-12-;/t;2-/m.0/s1
InChiKey:
LBWFXVZLPYTWQI-IPOVEDGCSA-N
Long Description:
Chemical. CAS: 341031-54-7. Formula: C22H27FN4O2 . C4H6O5. MW: 398.5 . 134.1. Potent ATP-competitive and cell permeable multi-targeted receptor tyrosine kinase (RTK) inhibitor targeting VEGFR and PDGFR-beta. Inhibits FLK1 (Ki=9nM), PDGFRbeta (Ki=8nM) and FLT3. It is at least 10-fold selective for FLK1 and PDGFRbeta over a variety of tyrosine kinases in a panel, including EGFR, Cdk2, Met, IGFR-1, Abl and Src. Inhibits the cellular receptor phosphorylation of FLT3, RET and CSF-1R. Also shown to inhibit c-Kit. Exhibits potent antiangiogenic and antitumor activity in multiple xenograft models.
MDL:
MFCD08282795
Molecular Formula:
C22H27FN4O2 . C4H6O5
Molecular Weight:
398.5 . 134.1
Package Type:
Plastic Vial
Precautions:
P260, P264, P281, P308 + P313, P405, P501
Product Description:
Potent ATP-competitive and cell permeable multi-targeted receptor tyrosine kinase (RTK) inhibitor targeting VEGFR and PDGFR-beta. Inhibits FLK1 (Ki=9nM), PDGFRbeta (Ki=8nM) and FLT3. It is at least 10-fold selective for FLK1 and PDGFRbeta over a variety of tyrosine kinases in a panel, including EGFR, Cdk2, Met, IGFR-1, Abl and Src. Inhibits the cellular receptor phosphorylation of FLT3, RET and CSF-1R. Also shown to inhibit c-Kit. Exhibits potent antiangiogenic and antitumor activity in multiple xenograft models.
Purity:
>98%
Signal word:
Danger
SMILES:
O[C@@H](CC(O)=O)C([O-])=O.[H][N+](CC)(CC)CCNC(=O)C1=C(C)NC(C=C2/C(=O)NC3=C2C=C(F)C=C3)=C1C
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or ethanol.
Transportation:
Non-hazardous
UNSPSC Category:
Protein Kinase Modulators
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase: L. Sun, et al.; J. Med. Chem. 46, 1116 (2003) | In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship: D. B. Mendel, et al.; Clin. Cancer Res. 9, 327 (2003) | SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo: A.M. O'Farrell, et al.; Blood 101, 3597 (2003) | Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors: J. Guo, et al.; Mol. Cancer. Ther. 5, 1007 (2006) | Molecular basis for sunitinib efficacy and future clinical development: S. Faivre, et al.; Nat. Rev. Drug Discov. 6, 734 (2007) | Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor: R. Roskoski; BBRC 356, 323 (2007)