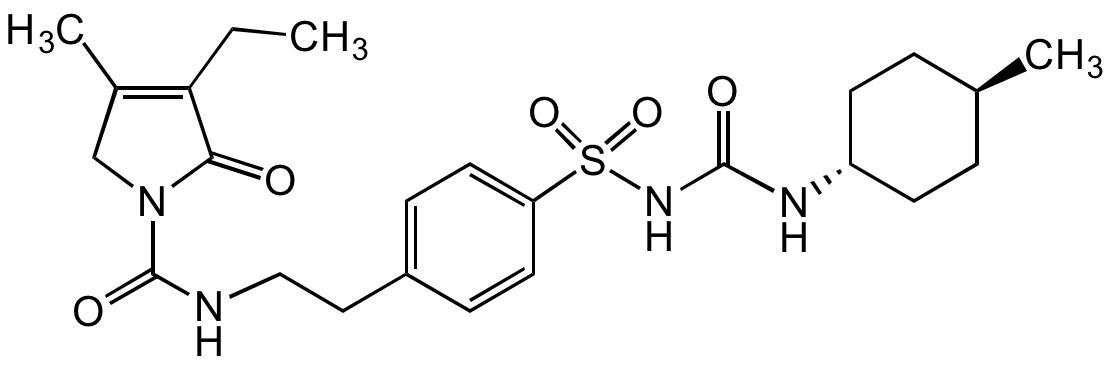
Glimepiride
| Code | Size | Price |
|---|
| CDX-G0208-M250 | 250 mg | £53.00 |
Quantity:
| CDX-G0208-G001 | 1 g | £145.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Antibody Isotype: n/a
Antibody Clone: n/a
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
trans-3-Ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[trans-4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxyamide; Amaryl; HOE 490
Appearance:
White solid.
CAS:
93479-97-1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H361
InChi:
InChI=1S/C24H34N4O5S/c1-4-21-17(3)15-28(22(21)29)24(31)25-14-13-18-7-11-20(12-8-18)34(32,33)27-23(30)26-19-9-5-16(2)6-10-19/h7-8,11-12,16,19H,4-6,9-10,13-15H2,1-3H3,(H,25,31)(H2,26,27,30)/t16-,19-
InChiKey:
WIGIZIANZCJQQY-RUCARUNLSA-N
Long Description:
Chemical. CAS: 93479-97-1. Formula: C24H34N4O5S. MW: 490.62. Glimepiride is a long-acting sulfonylurea antidiabetic agent inhibiting ATP-sensitive potassium (KATP) channels in pancreatic beta-cells, which leads to the release of insulin. In addition, Glimepiride increases the activity of intracellular insulin receptors. Studies conducted on adipocytes and skeletal muscle suggest that Glimepiride induces the PI3 kinase (PI3K) and Akt pathway, along with insulin receptor substrate-1/2 and endothelial nitric oxide synthase and stimulates glucose transporter 1 and 4 (GLUT1/4) expression. Glimepiride also increases osteoblast proliferation and differentiation, which is thought to be related to its ability to activate the PI3K and Akt pathway and exhibits neuroprotective benefit, decreasing expression and activity of BACE1 and amyloid-beta (Abeta) in neurons in a PPARgamma-dependent manner.
MDL:
MFCD00878417
Molecular Formula:
C24H34N4O5S
Molecular Weight:
490.62
Package Type:
Vial
Precautions:
P280
Product Description:
Glimepiride is a long-acting sulfonylurea antidiabetic agent inhibiting ATP-sensitive potassium (KATP) channels in pancreatic beta-cells, which leads to the release of insulin. In addition, Glimepiride increases the activity of intracellular insulin receptors. Studies conducted on adipocytes and skeletal muscle suggest that Glimepiride induces the PI3 kinase (PI3K) and Akt pathway, along with insulin receptor substrate-1/2 and endothelial nitric oxide synthase and stimulates glucose transporter 1 and 4 (GLUT1/4) expression. Glimepiride also increases osteoblast proliferation and differentiation, which is thought to be related to its ability to activate the PI3K and Akt pathway and exhibits neuroprotective benefit, decreasing expression and activity of BACE1 and amyloid-beta (Abeta) in neurons in a PPARgamma-dependent manner.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
O=C(N1C(C(CC)=C(C)C1)=O)NCCC2=CC=C(S(NC(N[C@H]3CC[C@H](C)CC3)=O)(=O)=O)C=C2
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or DMF (10mg/ml).
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at RT.
References
(1) W. Kramer, et al.; Horm. Metab. Res. 28, 464 (1996) | (2) G. Muller & K. Geisen; Horm. Metab. Res. 28, 469 (1996) | (3) D.K. Song & F.M. Ashcroft; Br. J. Pharmacol. 133, 193 (2001) | (4) M.L. Hribal, et al.; Mol. Pharmacol. 59, 322 (2001) | (5) C.L. Lawrence, et al.; Br. J. Pharmacol. 136, 746 (2002) | (6) K. Inukai, et al.; BBRC 328, 484 (2005) | (7) P. Ma, et al.; Metab. Clin. Exp. 59, 359 (2010) | (8) V.J. Briscoe, et al.; Expert Opin. Drug Metab. Toxicol. 6, 225 (2010) | (9) F. Liu, et al.; Neurosci. Lett. 557, 90 (2013)