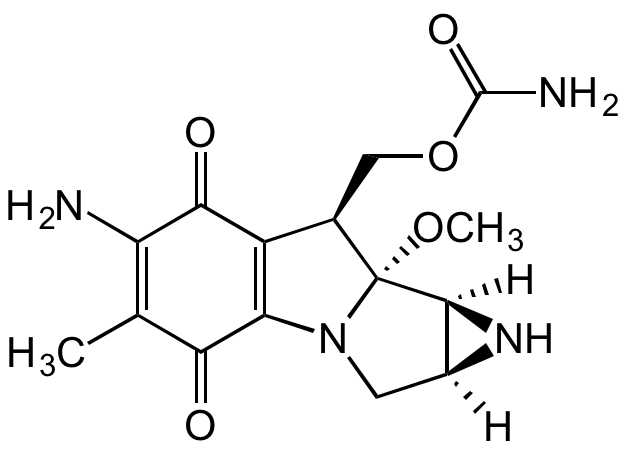
Mitomycin C
| Code | Size | Price |
|---|
| CDX-M0161-M005 | 5 mg | £59.00 |
Quantity:
| CDX-M0161-M010 | 10 mg | £96.00 |
Quantity:
| CDX-M0161-M025 | 25 mg | £169.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Antibody Isotype: n/a
Antibody Clone: n/a
Regulatory Status: RUO
Shipping:
Ambient
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Ametycine; Mutamycin; MMC; Mitocin-C; MitoExtra; Mitonco; Mitoplus; NSC 26980
Appearance:
Gray, purple or blue powder.
CAS:
50-07-7
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Protect from light and moisture.
Hazards:
H302-H351
InChi:
InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15?/m1/s1
InChiKey:
NWIBSHFKIJFRCO-BNVMVXHRSA-N
Long Description:
Chemical. CAS: 50-07-7. Formula: C15H18N4O5. MW: 334.33. Mitomycin C (MMC) is an antitumor antibiotic. This product is an alkylating agent that specifically targets the guanine nucleoside sequence 5'-CpG-3'. It inhibits DNA synthesis by covalently reacting with DNA, forming crosslinks between complementary strands of DNA. This interaction prevents separation of complementary DNA strands, inhibiting DNA replication. Mitomycin C causes the cross-linking of double-stranded DNA, which results in mutagenesis, inhibition of DNA synthesis, initiation of DNA repair events, and activation of apoptosis. In tissue culture, this compound decreases cell viability and suppresses mitosis, and causes disorganization of nucleus and the production of giant cells. Mitomycin C has strong antitumor activity, especially against Ehrlich ascites tumor cells, and strong bactericidal action against gram-positive and gram-negative bacteria. Mitomycin C from Streptomyces caespitosus has been used for the treatment of feeder layers such as, PMEF (primary mouse embryonic fibroblasts) and CD1 mouse embryonic fibroblasts (MEFs) for the culture of hESCs (human embryonic stem cells). It has also been used for the treatment of BLC (basal-like cancer) cell line.
MDL:
MFCD00078109
Molecular Formula:
C15H18N4O5
Molecular Weight:
334.33
Package Type:
Vial
Precautions:
P280-P301 + P312 + P330
Product Description:
Mitomycin C (MMC) is an antitumor antibiotic. This product is an alkylating agent that specifically targets the guanine nucleoside sequence 5'-CpG-3'. It inhibits DNA synthesis by covalently reacting with DNA, forming crosslinks between complementary strands of DNA. This interaction prevents separation of complementary DNA strands, inhibiting DNA replication. Mitomycin C causes the cross-linking of double-stranded DNA, which results in mutagenesis, inhibition of DNA synthesis, initiation of DNA repair events, and activation of apoptosis. In tissue culture, this compound decreases cell viability and suppresses mitosis, and causes disorganization of nucleus and the production of giant cells. Mitomycin C has strong antitumor activity, especially against Ehrlich ascites tumor cells, and strong bactericidal action against gram-positive and gram-negative bacteria. Mitomycin C from Streptomyces caespitosus has been used for the treatment of feeder layers such as, PMEF (primary mouse embryonic fibroblasts) and CD1 mouse embryonic fibroblasts (MEFs) for the culture of hESCs (human embryonic stem cells). It has also been used for the treatment of BLC (basal-like cancer) cell line.
Signal word:
Warning
SMILES:
NC1=C(C)C(C(N(C[C@@]2([H])[C@]3([H])N2)[C@@]3(OC)[C@@H]4COC(N)=O)=C4C1=O)=O
Solubility Chemicals:
Soluble in DMSO or DMF (20mg/ml). Sligthly soluble in water (1mg/ml).
Source / Host:
Isolated from microbial source.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) F.S. Philips, et al.; Cancer Res. 20, 1354 (1960) | (2) V.N. Iyer & W. Szybalski; PNAS 50, 355 (1963) | (3) M. Tomasz, et al.; Science 235, 1204 (1987) | (4) M. Tomasz, et al.; Biochemistry 27, 3182 (1988) | (5) M. Tomasz; Chem. Biol. 2, 575 (1995) | (6) G.R. Merlo, et al.; J. Cell Biol. 128, 1185 (1995) | (7) Y. Mao, et al.; Chem. Biol. 6, 251 (1999) | (8) Y. Kato, et al.; In Vivo 19, 301 (2005) | (9) V. Bryja, et al.; Nat. Protoc. 1, 2082 (2006) | (10) C.Y. Park, et al.; Nat. Protoc. 11, 2154 (2016)