S-Gboxin iodide
| Code | Size | Price |
|---|
| AG-CR1-3533-M001 | 1 mg | £105.00 |
Quantity:
| AG-CR1-3533-M005 | 5 mg | £390.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
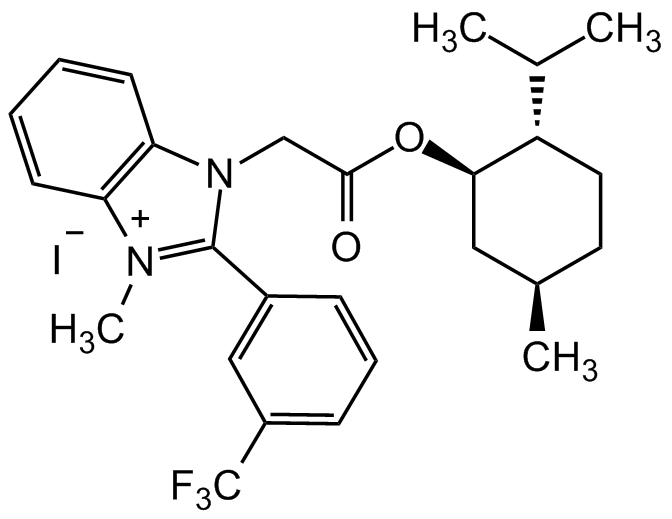
1-(2-(((1R,2S,5R)-2-Isopropyl-5-methylcyclohexyl)oxy)-2-oxoethyl)-3-methyl-2-(3-(trifluoromethyl)phenyl)-1H-benzo[d]imidazol-3-ium iodide
Appearance:
Solid.
CAS:
2101317-21-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C27H32F3N2O2.HI/c1-17(2)21-13-12-18(3)14-24(21)34-25(33)16-32-23-11-6-5-10-22(23)31(4)26(32)19-8-7-9-20(15-19)27(28,29)30;/h5-11,15,17-18,21,24H,12-14,16H2,1-4H3;1H/q+1;/p-1/t18-,21+,24-;/m1./s1
InChiKey:
DCAJNAWCJSUZDG-DZJKTSMVSA-M
Long Description:
Chemical. CAS: 2101317-21-7. Formula: C27H32F3N2O2 . I. MW: 473.6 . 126.9. Synthetic. Potent mitochondrial ATP synthase (ATPases (F0F1)) inhibitor, consequently leading to inhibition of oxidative phosphorylation (OXPHOS). Useful agent for immunometabolism research. Inhibits ATP synthase by blocking its proton channel (F0 subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production), significantly reducing electron flow through the electron transport chain. Potent antitumor agent that inhibits oxidative phosphorylation and consequently growth and proliferation of mouse and human glioblastoma (GBM) with an IC50 of 470nM. Shown to rapidly and irreversibly compromise oxygen consumption in glioblastoma cells. This functional Gboxin analog has excellent metabolic stability, enhanced plasma stability and pharmacokinetic properties, and is suitable for in vivo studies. Gboxin-resistant cells require a functional mitochondrial permeability transition pore that regulates pH and thus impedes the accumulation of Gboxin in the mitochondrial matrix. Administration of a metabolically stable Gboxin analog inhibits glioblastoma allografts and patient-derived xenografts. Gboxin toxicity extends to established human cancer cell lines of diverse organ origin and shows that the increased proton gradient and pH in cancer cell mitochondria is a mode of action that can be targeted in the development of antitumor reagents.
MDL:
MFCD32199204
Molecular Formula:
C27H32F3N2O2 . I
Molecular Weight:
473.6 . 126.9
Package Type:
Vial
Product Description:
Potent mitochondrial ATP synthase (ATPases (F0F1)) inhibitor, consequently leading to inhibition of oxidative phosphorylation (OXPHOS). Useful agent for immunometabolism research. Inhibits ATP synthase by blocking its proton channel (F0 subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production), significantly reducing electron flow through the electron transport chain. Potent antitumor agent that inhibits oxidative phosphorylation and consequently growth and proliferation of mouse and human glioblastoma (GBM) with an IC50 of 470nM. Shown to rapidly and irreversibly compromise oxygen consumption in glioblastoma cells. This functional Gboxin analog has excellent metabolic stability, enhanced plasma stability and pharmacokinetic properties, and is suitable for in vivo studies. Gboxin-resistant cells require a functional mitochondrial permeability transition pore that regulates pH and thus impedes the accumulation of Gboxin in the mitochondrial matrix. Administration of a metabolically stable Gboxin analog inhibits glioblastoma allografts and patient-derived xenografts. Gboxin toxicity extends to established human cancer cell lines of diverse organ origin and shows that the increased proton gradient and pH in cancer cell mitochondria is a mode of action that can be targeted in the development of antitumor reagents.
Purity:
>98% (1H-NMR)
SMILES:
CC(C)[C@@H]1CC[C@@H](C)C[C@H]1OC(CN2C(C=CC=C3)=C3[N+](C)=C2C4=CC(C (F)(F)F)=CC=C4)=O.[I-]
Solubility Chemicals:
Soluble in DMSO (50mg/ml), ethanol (50mg/ml) or water (2mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C.
References
Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma: Y. Shi, et al.; Nature 567, 341 (2019)