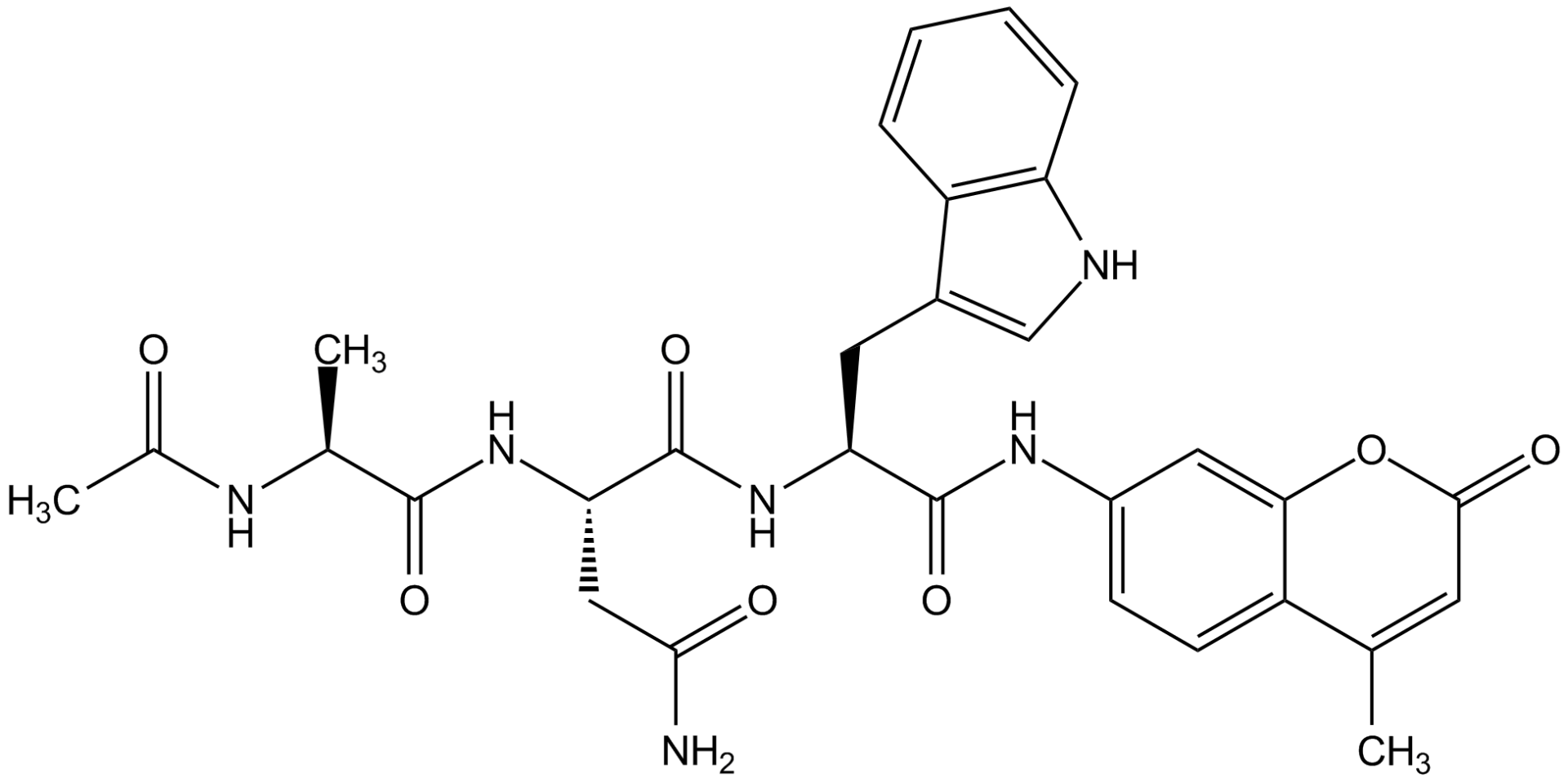
Ac-Ala-Asn-Trp-AMC
| Code | Size | Price |
|---|
| AG-CP3-0037-M001 | 1 mg | £53.00 |
Quantity:
| AG-CP3-0037-M005 | 5 mg | £97.00 |
Quantity:
| AG-CP3-0037-M025 | 25 mg | £358.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Further Information
Alternate Names/Synonyms:
Ac-ANW-AMC; Immunoproteasome Substrate
Appearance:
Lyophilized powder.
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Avoid freeze/thaw cycles.Protect from light.
InChi:
InChI=1S/C30H32N6O7/c1-15-10-27(39)43-25-12-19(8-9-20(15)25)34-29(41)23(11-18-14-32-22-7-5-4-6-21(18)22)36-30(42)24(13-26(31)38)35-28(40)16(2)33-17(3)37/h4-10,12,14,16,23-24,32H,11,13H2,1-3H3,(H2,31,38)(H,33,37)(H,34,41)(H,35,40)(H,36,42)/t16-,23-,24-/m0/s1
InChiKey:
ORTKHFLJSJQDKO-ZCWWJEROSA-N
Long Description:
Chemical. CAS: n/a. Formula: C30H32N6O7. MW: 588.6. Ac-ANW-AMC is a fluorogenic peptide substrate for measuring chymotrypsin-like activity of the immunoproteasome. Hydrolysis of this substrate by the beta5i-subunit of the immunoproteasome is monitored by observing fluorescence at an Excitation wavelength of 345nm and Emission at 445nm. This substrate is specific to the immunoproteasome and is not hydrolyzed efficiently by the constitutive proteasome.
Molecular Formula:
C30H32N6O7
Molecular Weight:
588.6
Other data:
Use: After preparing a stock solution in DMSO (>10mM) store product at -20°C to -80°C. It is recommended to make multiple aliquots after the first thaw to ensure best performance. Chymotrypsin-like activity can be measured using a typical working concentration range from 10-50µM.
Package Type:
Plastic Vial
Product Description:
Ac-ANW-AMC is a fluorogenic peptide substrate for measuring chymotrypsin-like activity of the immunoproteasome. Hydrolysis of this substrate by the beta5i-subunit of the immunoproteasome is monitored by observing fluorescence at an Excitation wavelength of 345nm and Emission at 445nm. This substrate is specific to the immunoproteasome and is not hydrolyzed efficiently by the constitutive proteasome.
Purity:
>97%
Sequence:
Acetyl-Ala-Asn-Trp-7-amido-4-methylcoumarin
SMILES:
O=C([C@@H](NC(C)=O)C)N[C@@H](CC(N)=O)C(N[C@@H](CC1=CNC2=C1C=CC=C2) C(NC3=CC=C(C(C)=CC(O4)=O)C4=C3)=O)=O
Solubility Chemicals:
Soluble in DMSO.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C.
References
Elucidating the catalytic subunit composition of distinct proteasome subtypes: A crosslinking approach employing bifunctional activity-based probes: C.K. Cornish, et al.; ChemBioChem 16, 284 (2015) | Pharmacodynamic monitoring of (immuno) proteasome inhibition during bortezomib treatment of a critically ill patient with lupus nephritis and myocarditis: K.A. De Groot, et al.; Lupus Sci. Med. 2, e000121 (2015) | Immunoproteasome beta5i-selective dipeptidomimetic inhibitors: P.K. Singh, et al.; ChemMedChem 11, 2127 (2016) | Acetaldehyde suppresses the display of HBV-MHC class I complexes on HBV-expressing hepatocytes: M. Ganesan, et al.; Am. J. Physiol. Gastrointest. Liver Physiol. 317, G127 (2019) | Monitoring the immunoproteasome in live cells using an activity-based peptide?peptoid hybrid probe: B.L. Zerfas & D.J. Trader; J. Am. Chem. Soc. 141, 5252 (2019)