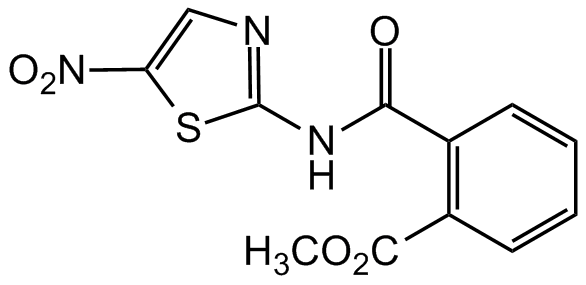
Nitazoxanide
| Code | Size | Price |
|---|
| AG-CR1-3723-M010 | 10 mg | £50.00 |
Quantity:
| AG-CR1-3723-M050 | 50 mg | £75.00 |
Quantity:
| AG-CR1-3723-M250 | 250 mg | £250.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
Ambient
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2-(Acetyloxy)-N-(5-nitro-2-thiazolyl)benzamide; NSC 697855; NTZ
Appearance:
White to off-white solid.
CAS:
55981-09-4
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Keep cool and dry.
Hazards:
H302, H315, H319, H335
InChi:
InChI=1S/C12H9N3O5S/c1-20-11(17)8-5-3-2-4-7(8)10(16)14-12-13-6-9(21-12)15(18)19/h2-6H,1H3,(H,13,14,16)
InChiKey:
GMMVCIKWTYKLKY-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 55981-09-4. Formula: C12H9N3O5S. MW: 307.3. Nitazoxanide is a broad-spectrum antiparasitic, antimicrobial and antiviral drug that is used in medicine for the treatment of various helminthic, protozoal, bacterial and viral infections. Nitazoxanide is rapidly metabolized to tizaxonide, an antiparasitic drug of the thiazolide class. The anti-protozoal activity and activity against anaerobic bacteria is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction which is essential to anaerobic energy metabolism. Nitazoxanide also showed a variety of other antibacterial mechanisms, inhibiting pyruvate dehydrogenase in E. coli, disrupting the membrane potential and pH homeostasis in the Mycobacterium tuberculosis and suppressing the chaperone/usher (CU) pathway of the Gram-negative bacteria. It is being studied for potential treatment for chronic hepatitis B, chronic hepatitis C, rotavirus, norovirus gastroenteritis and the coronavirus SARS-CoV-2 (COVID-19). The mechanism is suppressing the viral replication by inhibiting maturation of the viral hemagglutinin and the viral transcription factor immediate early 2 (IE2) as well as by activating the eukaryotic translation initiation factor 2alpha (an antiviral intracellular protein). Nitazoxanide modulates a variety of other pathways in vitro, including glutathione-S-transferase and glutamate-gated chloride ion channels in nematodes, respiration and other pathways in bacteria and cancer cells and viral and host transcriptional factors. Nitazoxanide has also been shown to be a potent antagonist of the Ca2+-activated Cl- channel TMEM16A, which offers a new mechanism to bronchodilate airways and block the multiple contractiles operating in severe diseases.
MDL:
MFCD00416599
Molecular Formula:
C12H9N3O5S
Molecular Weight:
307.3
Package Type:
Vial
Precautions:
P261, P264, P280, P301+P312, P302+352, P304+P340, P305+351+338, P405, P501
Product Description:
Nitazoxanide is a broad-spectrum antiparasitic, antimicrobial and antiviral drug that is used in medicine for the treatment of various helminthic, protozoal, bacterial and viral infections. Nitazoxanide is rapidly metabolized to tizaxonide, an antiparasitic drug of the thiazolide class. The anti-protozoal activity and activity against anaerobic bacteria is believed to be due to interference with the pyruvate:ferredoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction which is essential to anaerobic energy metabolism. Nitazoxanide also showed a variety of other antibacterial mechanisms, inhibiting pyruvate dehydrogenase in E. coli, disrupting the membrane potential and pH homeostasis in the Mycobacterium tuberculosis and suppressing the chaperone/usher (CU) pathway of the Gram-negative bacteria. It is being studied for potential treatment for chronic hepatitis B, chronic hepatitis C, rotavirus, norovirus gastroenteritis and the coronavirus SARS-CoV-2 (COVID-19). The mechanism is suppressing the viral replication by inhibiting maturation of the viral hemagglutinin and the viral transcription factor immediate early 2 (IE2) as well as by activating the eukaryotic translation initiation factor 2alpha (an antiviral intracellular protein). Nitazoxanide modulates a variety of other pathways in vitro, including glutathione-S-transferase and glutamate-gated chloride ion channels in nematodes, respiration and other pathways in bacteria and cancer cells and viral and host transcriptional factors. Nitazoxanide has also been shown to be a potent antagonist of the Ca2+-activated Cl- channel TMEM16A, which offers a new mechanism to bronchodilate airways and block the multiple contractiles operating in severe diseases.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
O=C(NC1=NC=C([N+]([O-])=O)S1)C2=C(C(OC)=O)C=CC=C2
Solubility Chemicals:
Soluble in DMSO (20mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Nitazoxanide: a new thiazolide antiparasitic agent: L.M. Fox & L.D. Saravolatz; Clin. Infect. Dis. 40, 1173 (2005) (Review) | Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni: P.S. Hoffman, et al.; Antimicrob. Agents Chemother. 51, 868 (2007) (Review) | Thiazolides, a New Class of Anti-Influenza Molecules Targeting Viral Hemagglutinin at the Post-Translational Level: J.F. Rossignol, et al.; J. Biol. Chem. 284, 29798 (2009) | Nitazoxanide kills replicating and nonreplicating Mycobacterium tuberculosis and evades resistance: L.P. de Carvalho, et al.; J. Med. Chem. 52, 5789 (2009) (Review) | Nitazoxanide inhibits biofilm formation by Staphylococcus epidermidis by blocking accumulation on surfaces: F. Tchouaffi-Nana, et al.; Antimicrob. Agents Chemother. 54, 2767 (2010) (Review) | Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracellular Ca(2+) stores: O. Ashiru, et al.; Virology 462-463, 135 (2014) (Review) | Nitazoxanide: a first-in-class broad-spectrum antiviral agent: J.F. Rossignol; Antiviral Res. 110, 94 (2014) (Review) | Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus: J.F. Rossignol; J. Infect. Public Health 9, 227 (2016) (Review) | Update on Nitazoxanide: A Multifunctional Chemotherapeutic Agent: A. Shakya, et al.; Curr. Drug Discov. Technol. 15, 201 (2018) (Review) | Nitazoxanide, an antiprotozoal drug, inhibits late-stage autophagy and promotes ING1-induced cell cycle arrest in glioblastoma: X. Wang, et al.; Cell Death Dis. 9, 1032 (2018) (Review) | Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways: K. Miner, et al.; Front. Pharmacol. 10, 51 (2019) (Review) | The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus: L.D. Jasenosky, et al.; iScience 19, 1279 (2019) (Review) | Astrovirus Replication Is Inhibited by Nitazoxanide In Vitro and In Vivo: V. Hargest, et al.; J. Virol. 94, e01706 (2020) (Review) | Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases: C. Liu, et al.; ACS Cent. Sci. 6, 315 (2020)