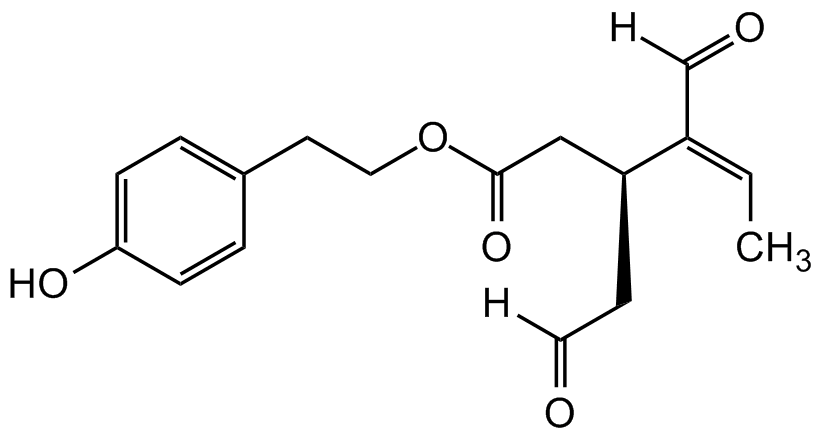
Oleocanthal
Product Code: AG-CN2-0537
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0537-M005 | 5 mg | £200.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(-)-Oleocanthal; 2-(4-Hydroxyphenyl)ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate; Deacetoxy Ligstroside Aglycon; p-HPEA-EDA
Appearance:
Amber oil.
CAS:
289030-99-5
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C17H20O5/c1-2-14(12-19)15(7-9-18)11-17(21)22-10-8-13-3-5-16(20)6-4-13/h2-6,9,12,15,20H,7-8,10-11H2,1H3/b14-2-/t15-/m0/s1
InChiKey:
VPOVFCBNUOUZGG-VAKDEWRISA-N
Long Description:
Chemical. CAS: 289030-99-5. Formula: C17H20O5. MW: 304.3. Oleocanthal is a naturally occurring amphiphilic secoiridoid from olive oil, with potent anti-inflammatory, antioxidant, anticancer and neuroprotective properties. Similar to classical non-steroidal anti-inflammatory drugs, it is a non-selective inhibitor of cyclooxygenase (COX-1, COX-2) enzymes in the prostaglandin biosynthesis. It also has been shown to inhibit the NLRP3 inflammasome. Inhibits proliferation, migration and invasion of various cancer cells while leaving healthy cells unharmed. It shows selective cytotoxicity, inducing lysosomal membrane permeabilization in cancer cells. It inhibits the enzymatic activity of mammalian target of rapamycin (mTOR), and the tyrosine kinase receptor c-Met (responsible for proliferation of many cell types) and Hsp90, consequently leading to cell cycle arrest during G1 phase. The neuroprotective activity includes the reduction of beta-amyloid protein accumulation via up-regulation of P-glycoprotein and LRP1, altering the fibrillization of tau proteins, key factors at the basis of neurodegenerative diseases and reducing oxidative stress. Activator of the TRPA1 ion channel, responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Shows antimicrobial activities.
MDL:
MFCD30543617
Molecular Formula:
C17H20O5
Molecular Weight:
304.3
Other data:
Note: Shown to react spontaneously with water or methanol to give mixtures of hemiacetals or acetals (see Karkoula et al 2012).
Package Type:
Vial
Product Description:
Oleocanthal is a naturally occurring amphiphilic secoiridoid from olive oil, with potent anti-inflammatory, antioxidant, anticancer and neuroprotective properties. Similar to classical non-steroidal anti-inflammatory drugs, it is a non-selective inhibitor of cyclooxygenase (COX-1, COX-2) enzymes in the prostaglandin biosynthesis. It also has been shown to inhibit the NLRP3 inflammasome. Inhibits proliferation, migration and invasion of various cancer cells while leaving healthy cells unharmed. It shows selective cytotoxicity, inducing lysosomal membrane permeabilization in cancer cells. It inhibits the enzymatic activity of mammalian target of rapamycin (mTOR), and the tyrosine kinase receptor c-Met (responsible for proliferation of many cell types) and Hsp90, consequently leading to cell cycle arrest during G1 phase. The neuroprotective activity includes the reduction of beta-amyloid protein accumulation via up-regulation of P-glycoprotein and LRP1, altering the fibrillization of tau proteins, key factors at the basis of neurodegenerative diseases and reducing oxidative stress. Activator of the TRPA1 ion channel, responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Shows antimicrobial activities.
Purity:
>97% (HPLC)
SMILES:
OC1=CC=C(CCOC(C[C@H](CC([H])=O)/C(C([H])=O)=CC)=O)C=C1
Solubility Chemicals:
Soluble in DMSO, dichloromethane or acetonitrile. Slightly soluble in methanol, ethanol or water (0.1mg/ml).
Source / Host:
Isolated from Olea europaea.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives: G. Montedoro, et al.; J. Agric. Food Chem. 41, 2228 (1993) | Phytochemistry: ibuprofen-like activity in extra-virgin olive oil: G.K. Beauchamp, et al.; Nature 437, 7055 (2005) | Synthesis and assignment of absolute configuration of (-)-oleocanthal: a potent, naturally occurring non-steroidal anti-inflammatory and anti-oxidant agent derived from extra virgin olive oils: A.B. Smith, et al.; Org. Lett. 7, 5075 (2005) | Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity: E. Medina, et al.; J. Agric. Food Chem. 54, 4954 (2006) | Sensory characterization of the irritant properties of oleocanthal, a natural anti-inflammatory agent in extra virgin olive oils: S. Cicerale, et al.; Chem. Senses 34, 333 (2009) | (-)-Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancers: A.Y. Elnagar, et al.; Planta Med. 77, 1013 (2011) | Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuroprotective mechanism against Alzheimer's disease: in vitro and in vivo studies: A.H. Abuznait, et al.; ACS Chem. Neurosci. 4, 973 (2013) | Modulation of tau protein fibrillization by oleocanthal: M.C. Monti, et al.; J. Nat. Prod. 75, 1584 (2012) | Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative 1H NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils: E. Karkoula, et al.; J. Agric. Food Chem. 60, 11696 (2012) | Chemical proteomics-driven discovery of oleocanthal as an Hsp90 inhibitor: L. Margarucci, et al.; Chem. Commun. 49, 5844 (2013) | Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models: M.R. Akl, et al.; PLoS One 9, e97622 (2014) | New drugs from ancient natural foods. Oleocanthal, the natural occurring spicy compound of olive oil: a brief history: M. Scotece, et al.; Drug Discov. Today 20, 406 (2015) (Review) | Olive Oil-derived Oleocanthal as Potent Inhibitor of Mammalian Target of Rapamycin: Biological Evaluation and Molecular Modeling Studies: M.A. Khanfar, et al.; Phytother. Res. 29, 1776 (2015) | Current Disease-Targets for Oleocanthal as Promising Natural Therapeutic Agent: A. Segura-Carretero & J.A. Curiel; Int. J. Mol. Sci. 19, E2899 (2018) (Review) | New neuroprotective perspectives in fighting oxidative stress and improving cellular energy metabolism by oleocanthal: C. Angeloni, et al.; Neural. Regen. Res. 14, 1217 (2019) | Oleocanthal-Rich Extra-Virgin Olive Oil Restores the Blood-Brain Barrier Function through NLRP3 Inflammasome Inhibition Simultaneously with Autophagy Induction in TgSwDI Mice: S.B. Al Rihani, et al.; ACS Chem. Neurosci. 10, 3543 (2019) | (-)-Oleocanthal and (-)-oleocanthal-rich olive oils induce lysosomal membrane permeabilization in cancer cells: L. Goren, et al.; PLoS One 14, e0216024 (2019)