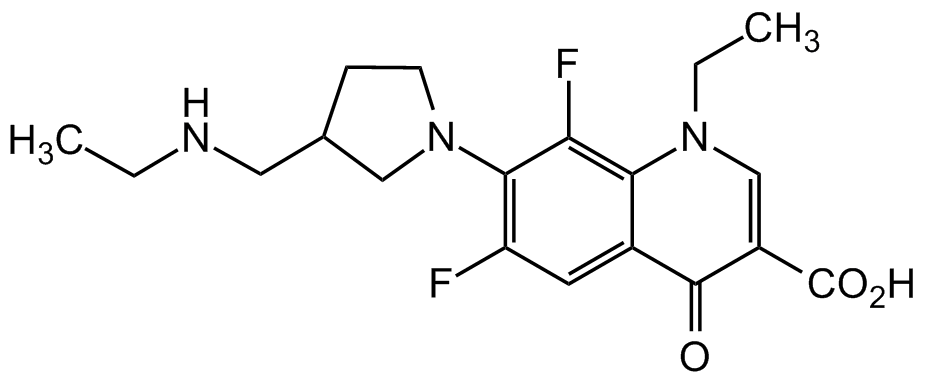
Merafloxacin
| Code | Size | Price |
|---|
| AG-CR1-3756-M005 | 5 mg | £80.00 |
Quantity:
| AG-CR1-3756-M025 | 25 mg | £290.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
CI-934; PD 114843; 1-Ethyl-7-(3-((ethylamino)methyl)-1-pyrrolidinyl)-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid
Appearance:
White to off-white solid.
CAS:
91188-00-0
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C19H23F2N3O3/c1-3-22-8-11-5-6-24(9-11)17-14(20)7-12-16(15(17)21)23(4-2)10-13(18(12)25)19(26)27/h7,10-11,22H,3-6,8-9H2,1-2H3,(H,26,27)
InChiKey:
BAYYCLWCHFVRLV-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 91188-00-0. Formula: C19H23F2N3O3. MW: 379.4. Merafloxacin is a fluoroquinolone broad-spectrum antibacterial compound. It is an inhibitor of bacterial DNA gyrase and Type II DNA topoisomerase. It demonstrated excellent activity against Gram-positive organisms and less potent activity against Gram-negative bacteria.
Merafloxacin is a programmed -1 ribosomal frameshifting (-1 PRF) inhibitor of SARS-CoV-2. Frameshift inhibition by merafloxacin is robust to mutations within the pseudoknot region and is similarly effective on -1 PRF of other betacoronaviruses and blocks SARS-CoV-2 replication in Vero E6 cells. The compound reduced viral levels in infected African green monkey VeroE6 cells in a concentration-dependent manner. Merafloxacin showed no cellular toxicity and resulted in a 3 to 4 orders of magnitude reduction of SARS-CoV-2 titer, with IC50 of 4.3 µMu. This compound provides a starting point for high-throughput screening for frameshifting inhibitors as a viable target for therapeutic intervention against SARS-CoV-2.
MDL:
MFCD00864816
Molecular Formula:
C19H23F2N3O3
Molecular Weight:
379.4
Package Type:
Vial
Product Description:
Merafloxacin is a fluoroquinolone broad-spectrum antibacterial compound. It is an inhibitor of bacterial DNA gyrase and Type II DNA topoisomerase. It demonstrated excellent activity against Gram-positive organisms and less potent activity against Gram-negative bacteria. Merafloxacin is a programmed -1 ribosomal frameshifting (-1 PRF) inhibitor of SARS-CoV-2. Frameshift inhibition by merafloxacin is robust to mutations within the pseudoknot region and is similarly effective on -1 PRF of other betacoronaviruses and blocks SARS-CoV-2 replication in Vero E6 cells. The compound reduced viral levels in infected African green monkey VeroE6 cells in a concentration-dependent manner. Merafloxacin showed no cellular toxicity and resulted in a 3 to 4 orders of magnitude reduction of SARS-CoV-2 titer, with IC50 of 4.3 µMu. This compound provides a starting point for high-throughput screening for frameshifting inhibitors as a viable target for therapeutic intervention against SARS-CoV-2.
Purity:
>98% (NMR)
SMILES:
O=C1C2=CC(F)=C(N3CCC(CNCC)C3)C(F)=C2N(CC)C=C1C(O)=O
Solubility Chemicals:
Soluble in DMSO.
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
In vitro activity of CI-934, a quinolone carboxylic acid active against gram-positive and -negative bacteria: M.A. Cohen, et al.; Antimicrob. Agents Chemother. 28, 766 (1985) | Discrepancy between the antibacterial activities and the inhibitory effects on Micrococcus luteus DNA gyrase of 13 quinolones: K.P. Fu, et al.; Chemotherapy 32, 494 (1986) | In vitro activity of CI-934 and other antimicrobial agents against gram-positive and gram-negative bacteria: R.P. Smith, et al.; Clin. Ther. 9, 106 (1986) | In vitro assessment of CI-934-a new quinolone derivative: R. Finch, et al.; Chemioterapia 5, 368 (1986) | Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus: K. Hoshino, et al.; Antimicrob. Agents Chemother. 33, 1816 (1989) | Restriction of SARS-CoV-2 Replication by Targeting Programmed -1 Ribosomal Frameshifting In Vitro: Y. Sun, et al.; Preprint (2020) | Programmed ?1 Ribosomal Frameshifting in coronaviruses: A therapeutic target: J.A. Kelly, et al.; Virology 554, 75 (2021) | Structural basis of ribosomal frameshifting during translation of the SARS-CoV-2 RNA genome: P.R. Bhatt, et al.; Science (Epub ahead of print) (2021)