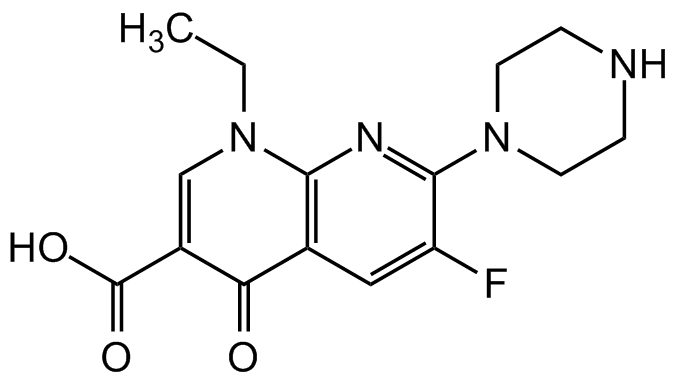
Enoxacin
| Code | Size | Price |
|---|
| AG-CR1-3539-M050 | 50 mg | £40.00 |
Quantity:
| AG-CR1-3539-M250 | 250 mg | £100.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +4°C, Long Term: -20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
NSC 629661; PD 107779; CI 919; AT 2266; BRN 3628995; Abenox; Bactidan; Comprecin; Flumark; Penetrex
Appearance:
White to off-white solid.
CAS:
74011-58-8
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.
InChi:
InChI=1S/C15H17FN4O3/c1-2-19-8-10(15(22)23)12(21)9-7-11(16)14(18-13(9)19)20-5-3-17-4-6-20/h7-8,17H,2-6H2,1H3,(H,22,23)
InChiKey:
IDYZIJYBMGIQMJ-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 74011-58-8. Formula: C15H17FN4O3. MW: 320.3. Enoxacin is a cell permeable fluoroquinolone broad-spectrum antibacteral agent. It is active against clinical isolates of a variety of Gram-positive and Gram-negative bacteria. Fluoroquinolones are a group of broad-spectrum antibiotics that inhibit DNA synthesis. They prevent unwinding of double-stranded DNA by binding DNA gyrase or DNA topoisomerase IV, prevent the formation of single-stranded DNA and inhibit bacterial replication. Enoxacin is used to treat urinary tract infections and gonorrhea. Osteoclastogenesis inhibitor. Shown to decrease RANKL-induced JNK signaling and inhibiting osteoclast formation. Additionally, Enoxacin inhibits vacuolar H+ ATPase activity, preventing bone resorption. Enoxacin is a unique small molecule enhancer of microRNA (SMER) maturation. MicroRNAs (miRNAs) are small RNA molecules that regulate gene expression at the posttranscriptional level and are critical for many cellular pathways. The disruption of miRNAs and their processing machineries also contributes to the development of human tumors. The RNA interference (RNAi) represents the foundation underlying complex biological mechanisms that are dysregulated in many diseases. Enoxacin exerts an antiviral activity and could be a promising candidate for COVID-19 treatment through enhancing the RNAi pathway as an inflammation-free innate immune defense against viral infections and through inhibition of viral helicases. Shows antiviral activity against SARS-CoV-2 and MERS-CoV. Enoxacin enhances the production of miRNAs with tumor suppressor functions by binding to the miRNA biosynthesis protein TAR RNA-binding protein 2 (TRBP). Restoring the global expression of miRNAs by Enoxacin has been attributed to its anti-tumor properties. Enoxacin counteracts obesity by promoting thermogenic signaling and inducing oxidative metabolism in adipose tissue and skeletal muscle through miRNA-mediated regulation. Enoxacin increased lifespan in C. elegans by inhibiting miR-34-5p levels, interfering with the redox balance and promoting healthspan.
MDL:
MFCD00133308
Molecular Formula:
C15H17FN4O3
Molecular Weight:
320.3
Package Type:
Vial
Product Description:
Enoxacin is a cell permeable fluoroquinolone broad-spectrum antibacterial agent. It is active against clinical isolates of a variety of Gram-positive and Gram-negative bacteria. Fluoroquinolones are a group of broad-spectrum antibiotics that inhibit DNA synthesis. They prevent unwinding of double-stranded DNA by binding DNA gyrase or DNA topoisomerase IV, prevent the formation of single-stranded DNA and inhibit bacterial replication. Enoxacin is used to treat urinary tract infections and gonorrhea. Osteoclastogenesis inhibitor. Shown to decrease RANKL-induced JNK signaling and inhibiting osteoclast formation. Additionally, Enoxacin inhibits vacuolar H+ ATPase activity, preventing bone resorption. Enoxacin is a unique small molecule enhancer of microRNA (SMER) maturation. MicroRNAs (miRNAs) are small RNA molecules that regulate gene expression at the posttranscriptional level and are critical for many cellular pathways. The disruption of miRNAs and their processing machineries also contributes to the development of human tumors. The RNA interference (RNAi) represents the foundation underlying complex biological mechanisms that are dysregulated in many diseases. Enoxacin exerts an antiviral activity and could be a promising candidate for COVID-19 treatment through enhancing the RNAi pathway as an inflammation-free innate immune defense against viral infections and through inhibition of viral helicases. Shows antiviral activity against SARS-CoV-2 and MERS-CoV. Enoxacin enhances the production of miRNAs with tumor suppressor functions by binding to the miRNA biosynthesis protein TAR RNA-binding protein 2 (TRBP). Restoring the global expression of miRNAs by Enoxacin has been attributed to its anti-tumor properties. Enoxacin counteracts obesity by promoting thermogenic signaling and inducing oxidative metabolism in adipose tissue and skeletal muscle through miRNA-mediated regulation. Enoxacin increased lifespan in C. elegans by inhibiting miR-34-5p levels, interfering with the redox balance and promoting healthspan.
Purity:
>98%
SMILES:
CCN(C=C(C(O)=O)C1=O)C2=C1C=C(F)C(N3CCNCC3)=N2
Solubility Chemicals:
Soluble in NaOH (50mg/ml), DMSO (1mg/ml) or water (1mg/ml).
Transportation:
Non-hazardous
UNSPSC Category:
Biochemical Reagents
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
In-vitro activity of enoxacin (CL-919), a new quinoline derivative, compared with that of other antimicrobial agents: R. Wise, et al.; J. Antimicrob. Chemother. 13, 237 (1984) | Enoxacin: worldwide in-vitro activity against 22451 clinical isolates: C. Siporin & G. Towse; J. Antimicrob. Chemother. 14, 47 (1984) | In-vitro activity of pefloxacin compared to enoxacin, norfloxacin, gentamicin and new beta-lactams: A.M. Clarke, et al.; J. Antimicrob. Chemother. 15, 39 (1985) | Enoxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use: J.M. Henwood & J.P. Monk; Drugs 36, 32 (1988) (Review) | Antimicrobial activity of enoxacin: G. Nicoletti, et al.; J. Chemother. 1, 143 (1989) | Anti-staphylococcal activity of temafloxacin, ciprofloxacin, ofloxacin and enoxacin: A.L. Barry & P.C. Fuchs; J. Antimicrob. Chemother. 28, 695 (1991) | The DNA cleavage reaction of DNA gyrase. Comparison of stable ternary complexes formed with enoxacin and CcdB protein: K.E. Scheirer & N.P. Higgins; J. Biol. Chem. 272, 27202 (1997) | Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition: M. Takei, et al.; Antimicrob. Agents Chemother. 45, 3544 (2001) | Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing: S. Melo, et al.; PNAS 108, 4394 (2011) | Enoxacin directly inhibits osteoclastogenesis without inducing apoptosis: E.J. Toro, et al.; J. Biol. Chem. 287, 17894 (2012) | Enoxacin inhibits growth of prostate cancer cells and effectively restores microRNA processing: E. Sousa, et al.; Epigenetics 8, 548 (2013) | Enoxacin extends lifespan of C. elegans by inhibiting miR-34-5p and promoting mitohormesis: S. Pinto, et al.; Redox Biol. 18, 84 (2018) | Simian Virus 40 Large T Antigen as a Model to Test the Efficacy of Flouroquinolones against Viral Helicases: S. Siddiqui, et al.; Bioinformation 14, 75 (2018) | Enoxacin inhibits proliferation and invasion of human osteosarcoma cells and reduces bone tumour volume in a murine xenograft model: X. Luo, et al.; Oncol. Lett. 20, 1400 (2020) | Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV: S.L.P. Scroggs, et al.; Viruses 13, 8 (2020) | Modulating microRNA Processing: Enoxacin, the Progenitor of a New Class of Drugs: T. Felicetti, et al.; J. Med. Chem. 63, 12275 (2020) | Enoxacin induces oxidative metabolism and mitigates obesity by regulating adipose tissue miRNA expression: A.L. Rocha, et al.; Sci. Adv. 6, eabc6250 (2020) | In silico analysis suggests the RNAi-enhancing antibiotic enoxacin as a potential inhibitor of SARS-CoV-2 infection: A. Ahmadi & S. Moradi; Sci. Rep. 11, 10271 (2021) | Targeting miRNA dysregulation with small molecules: the case of enoxacin: T. Felicetti & G. Manfroni; Future Med. Chem. 13, 1245 (2021)