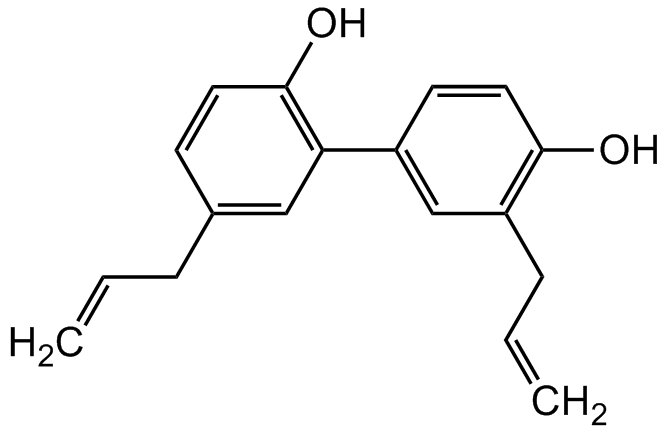
Honokiol
| Code | Size | Price |
|---|
| CDX-H0139-M010 | 10 mg | £84.00 |
Quantity:
| CDX-H0139-M025 | 25 mg | £157.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
5,3'-Diallyl-2,4'-dihydroxybiphenyl; NSC 293100
Appearance:
Light brown powder.
CAS:
35354-74-6
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C18H18O2/c1-3-5-13-7-9-18(20)16(11-13)14-8-10-17(19)15(12-14)6-4-2/h3-4,7-12,19-20H,1-2,5-6H2
InChiKey:
FVYXIJYOAGAUQK-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 35354-74-6. Formula: C18H18O2. MW: 266.33. Honokiol is a natural product derived from parts of the plant M. grandiflora used in Oriental herbal medicine. It is a lignan with diverse biological activities, including anticancer, neuroprotective, antithrombotic, anti-inflammatroy, antioxidant, antiviral, analgesic, antimicrobial, metabolic and GABA-modulating properties. Honokiol exerts broad-range anticancer activity in vitro and in vivo such as induction of cell cycle arrest and apoptosis, inhibition of epithelial-mesenchymal transition, inhibiting cell migration, invasion, and metastasis, as well as inducing anti-angiogenesis activity. Honokiol has neuroprotective effects based on its potent antioxidant activity, blockade of glutamate receptors and reduction in neuroinflammation. Honokiol also blocks inflammatory factor production in glial cells through the inhibition on NF-kappa activation. It protects against lipid peroxidation by interfering with ROS production and migration. It regulates gamma-aminobutyric acid (GABA) A receptors and it also prevents platelet aggregation. Honokiol has been shown to inhibit hepatitis C virus (HCV) infection in vitro and described as a non-adipogenic PPARgamma agonist.
MDL:
MFCD00016674
Molecular Formula:
C18H18O2
Molecular Weight:
266.33
Package Type:
Vial
Product Description:
Honokiol is a natural product derived from parts of the plant M. grandiflora used in Oriental herbal medicine. It is a lignan with diverse biological activities, including anticancer, neuroprotective, antithrombotic, anti-inflammatroy, antioxidant, antiviral, analgesic, antimicrobial, metabolic and GABA-modulating properties. Honokiol exerts broad-range anticancer activity in vitro and in vivo such as induction of cell cycle arrest and apoptosis, inhibition of epithelial-mesenchymal transition, inhibiting cell migration, invasion, and metastasis, as well as inducing anti-angiogenesis activity. Honokiol has neuroprotective effects based on its potent antioxidant activity, blockade of glutamate receptors and reduction in neuroinflammation. Honokiol also blocks inflammatory factor production in glial cells through the inhibition on NF-kappa activation. It protects against lipid peroxidation by interfering with ROS production and migration. It regulates gamma-aminobutyric acid (GABA) A receptors and it also prevents platelet aggregation. Honokiol has been shown to inhibit hepatitis C virus (HCV) infection in vitro and described as a non-adipogenic PPARgamma agonist.
Purity:
>98% (HPLC)
SMILES:
OC1=C(C2=CC(CC=C)=C(O)C=C2)C=C(CC=C)C=C1
Solubility Chemicals:
Soluble in DMSO (30mg/ml), DMF (30mg/ml) or ethanol (30mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) Y. Fukuyama, et al.; Bioorg. Med. Chem. Lett. 12, 1163 (2002) | (2) K.-T. Liou, et al.; Brain Res 992, 159 (2003) | (3) X. Bai, et al.; J. Biol. Chem. 278, 35501 (2003) | (4) H. Hu, et al.; Acta Pharmacol. Sin. 26, 1063 (2005) | (5) K.S. Ahn, et al.; Mol. Cancer Res. 4, 621 (2006) | (6) S. Dikalov, et al.; Biochem. Pharmacol. 76, 589 (2008) | (7) B.H. Kim J.Y. Cho; Acta. Pharmacol. Sin. 29, 113 (2008) | (8) L.E. Fried & J.L. Arbiser; Antioxid. Redox Signal. 11, 1139 (2009) (Review) | (9) L.K. Chao, et al.; J. Agric. Food Chem. 58, 3472 (2010) | (10) J.L. Shen, et al.; Molecules 15, 6452 (2010) (Review) | (11) K.H. Lan, et al.; Liver Internat. 32, 989 (2012) | (12) A.G. Atanasov, et al.; Biochim. Biophys. Acta 1830, 4813 (2013) | (13) A. Kumar, et al.; Future Med. Chem. 5, 809 (2013) (Review) | (14) A. Woodbury, et al.; Front. Neurol. 4, 130 (2013) (Review) | (15) S. Talarek, et al.; Biofactors 43, 760 (2017) (Review) | (16) C.P. Ong, et al.; Cancers 12, E48 (2019) (Review