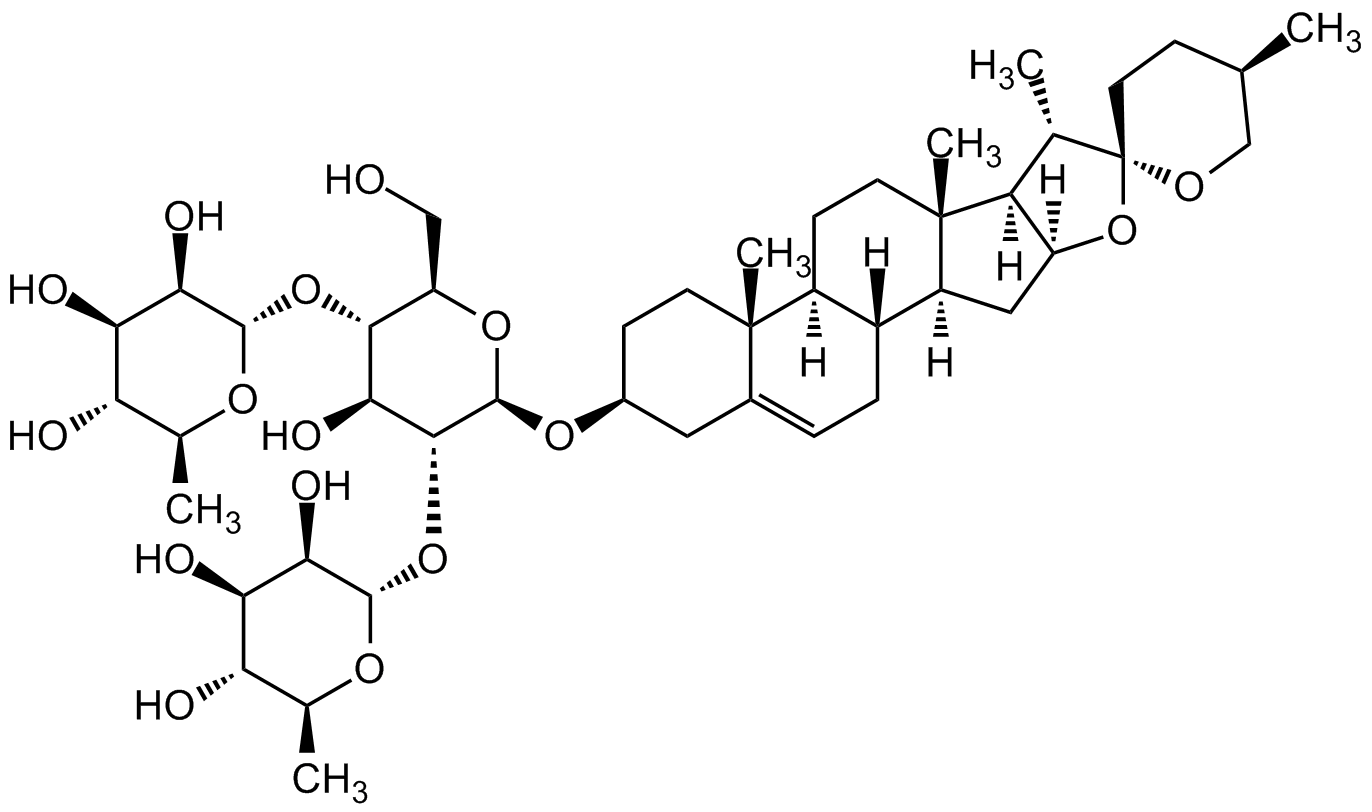
Dioscin
| Code | Size | Price |
|---|
| CDX-D0195-M025 | 25 mg | £218.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Collettiside III; Polyphyllin III; CCRIS 4123
Appearance:
Wthie to off-white powder.
CAS:
19057-60-4
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C45H72O16/c1-19-9-14-45(54-18-19)20(2)30-28(61-45)16-27-25-8-7-23-15-24(10-12-43(23,5)26(25)11-13-44(27,30)6)57-42-39(60-41-36(52)34(50)32(48)22(4)56-41)37(53)38(29(17-46)58-42)59-40-35(51)33(49)31(47)21(3)55-40/h7,19-22,24-42,46-53H,8-18H2,1-6H3/t19-,20+,21+,22+,24+,25-,26+,27+,28+,29-,30+,31+,32+,33-,34-,35-,36-,37+,38-,39-,40+,41+,42-,43+,44+,45-/m1/s1
InChiKey:
VNONINPVFQTJOC-ZGXDEBHDSA-N
Long Description:
Chemical. CAS: 19057-60-4. Formula: C45H72O16. MW: 869.04. Dioscin is a natural steroid saponin originally isolated from plants used in herbal remedies. It has anticancer, anti-inflammatory, immunoregulatory, antioxidant, hyoplipidemic, antiviral, neuroprotective and antifungal activities. It inhibits proliferation, induces autophagy and oxygen-mediated apoptosis in cancer cells. It has been shown to inhibit colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK pathways. It inhibits adipogenesis through the AMPK/MAPK pathway. Dioscin showed protective effect against liver fibrosis by potently inhibiting ITGA5. Promotes proliferation of pancreatic beta cells via Wnt/beta-catenin signaling pathways. It has been shown inhibit the nuclear transport of NF-kappa and the expression of reactive oxygen species (ROS) and consequently the expression of inflammasome targets such as NLRP3. Potent inhibitor of Skp2, component of the ubiquitin/proteasome degradation pathway.
MDL:
MFCD02094174
Molecular Formula:
C45H72O16
Molecular Weight:
869.04
Package Type:
Vial
Product Description:
Dioscin is a natural steroid saponin originally isolated from plants used in herbal remedies. It has anticancer, anti-inflammatory, immunoregulatory, antioxidant, hyoplipidemic, antiviral, neuroprotective and antifungal activities. It inhibits proliferation, induces autophagy and oxygen-mediated apoptosis in cancer cells. It has been shown to inhibit colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK pathways. It inhibits adipogenesis through the AMPK/MAPK pathway. Dioscin showed protective effect against liver fibrosis by potently inhibiting ITGA5. Promotes proliferation of pancreatic beta cells via Wnt/beta-catenin signaling pathways. It has been shown inhibit the nuclear transport of NF-kappa and the expression of reactive oxygen species (ROS) and consequently the expression of inflammasome targets such as NLRP3. Potent inhibitor of Skp2, component of the ubiquitin/proteasome degradation pathway.
Purity:
>98% (NMR)
SMILES:
C[C@H](CC1)CO[C@]21O[C@@]3([H])C[C@@]4([H])[C@]5([H])CC=C6C[C@@H](O[C@H](O[C@@H]7CO)[C@H](O[C@H]8[C@H](O)[C@H](O)[C@@H](O)[C@H](C)O8)[C@@H](O)[C@@H]7O[C@@H]9O[C@@H](C)[C@H](O)[C@@H](O)[C@H]9O)CC[C@]6(C)[C@@]5([H])CC[C@]4(C)[C@@]3([H])[C@@H]2C
Solubility Chemicals:
Soluble in DMSO (10mg/ml), DMF (10mg/ml) or ethanol (5mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) J. Cai, et al.; Biol. Pharm. Bull. 25, 193 (2002) | (2) Z. Wang, et al.; Cancer Biol. Ther. 13, 138 (2012) | (3) J. cho, et al.; Biochim. Biophys. Acta. 1828, 1153 (2013) | (4) M.J. Hsieh, et al.; Arch. Toxicol. 87, 1927 (2013) | (5) L. Lv, et al.; Food Chem. Toxicol. 59, 657 (2013) | (6) B. Poudel, et al.; Int. J. Mol. Med. 34, 1401 (2014) | (7) S. Wu, et al.; Biochimie 110, 62 (2015) | (8) M. Liu, et al.; Sci. Rep. 5, 7973 (2015) | (9) L. Xu, et al.; Food Chem. Toxicol. 107, 318 (2017) | (10) F. Yu, et al.; Clin. Lab. 64, 785 (2018) | (11) X. Tao, et al.; Pharmacol. Res. 137, 259 (2018) (Review) | (12) Y. Qi, et al.; Molecules 24, E1247 (2019) | (13) L. Yang, et al.; Biomed. Res. Int. 2019, 5763602 (2019) (Review) | (14) W. Yin, et al.; Small 16, e1905977 (2020) | (15) W. Jiang, et al.; EBioMedicine 51, 102593 (2020)