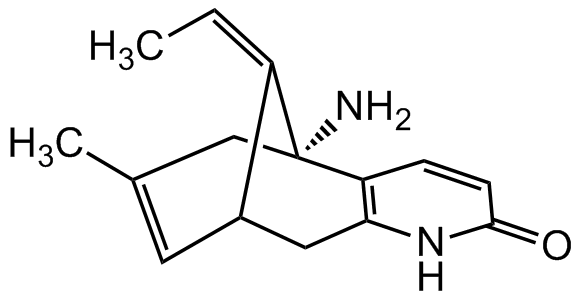
(-)-Huperzine A
Product Code:
CDX-H0208
CDX-H0208
Host Type:
Plant
Plant
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
+4°C
+4°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-H0208-M025 | 25 mg | £121.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Show All
Further Information
Alternate Names/Synonyms:
Hup A; (-)-Selagine; Kimpukan A
Appearance:
White to off-white powder.
CAS:
102518-79-6
Class:
6.1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H300
InChi:
InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/t10-,15+/m0/s1
InChiKey:
ZRJBHWIHUMBLCN-ZUZCIYMTSA-N
Long Description:
Chemical. CAS: 102518-79-6. Formula: C15H18N2O. MW: 242.32. Huperzine A is a natural sesquiterpene alkaloid. It showns neuroprotective activity. It is a potent acetylcholinesterase (AChE) inhibitor and displays oral AChE inhibitory activity in animals. Huperzine A has potential applications in a variety of neuroprotective roles, including protection against organophosphate nerve agents and ameliorating symptoms of Alzheimer?s disease. It has been shown to reduce neuronal cell death caused by glutamate and to have anti-inflammatory effects. It also has been shown to be an antagonist of NMDA receptor in cerebral cortex. It has been shown to ameliorate cognitive deficits.
MDL:
MFCD01714949
Molecular Formula:
C15H18N2O
Molecular Weight:
242.32
Package Type:
Vial
PG:
II
Product Description:
Huperzine A is a natural sesquiterpene alkaloid. It showns neuroprotective activity. It is a potent acetylcholinesterase (AChE) inhibitor and displays oral AChE inhibitory activity in animals. Huperzine A has potential applications in a variety of neuroprotective roles, including protection against organophosphate nerve agents and ameliorating symptoms of Alzheimer?s disease. It has been shown to reduce neuronal cell death caused by glutamate and to have anti-inflammatory effects. It also has been shown to be an antagonist of NMDA receptor in cerebral cortex. It has been shown to ameliorate cognitive deficits.
Purity:
>98% (HPLC)
Signal Word:
Danger
SMILES:
C/C=C1[C@@H]2CC3=C(C=CC(N3)=O)[C@@]/1(N)CC(C)=C2
Solubility Chemicals:
Soluble in DMSO (20mg/ml) or methanol (1mg/ml).
Source / Host:
Plant
Transportation:
Excepted Quantity
UN Nummer:
1544PSN1
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
Documents
References
(1) S.J. Geib, et al.; Acta Crystallogr. C. 47, 824 (1991) | (1) H.S. Ved, et al.; Neuroreport 8, 963 (1997) | (1) J.M. Zhang & G.Y. Hu; Neuroscience 105, 663 (2001) | (1) R.K. Gordon, et al.; J. Appl. Toxicol. 21, S47 (2001) | (1) J. Zhou & X.C. Tang; FEBS Lett. 526, 21 (2002) | (1) R. Wang & X.C. Tang; Neurosignals 14, 71 (2005) (Review) | (1) H.Y. Zhang X.C. Tang; Trends Pharmacol. Sci. 27, 619 (2006) (Review) | (1) Z.F. Wang, et al.; J. Neurochem. 106, 1594 (2008) | (1) X.Y. Mao, et al.; Int. J. Mol. Sci. 15, 7667 (2014) | (1) H.Y. Zhang, et al.; Cell Mol. Neurobiol. 28, 173 (2008) (Review) | (1) U. Damar, et al.; Expert Rev. Neurother. 16, 671 (2016) (Review)