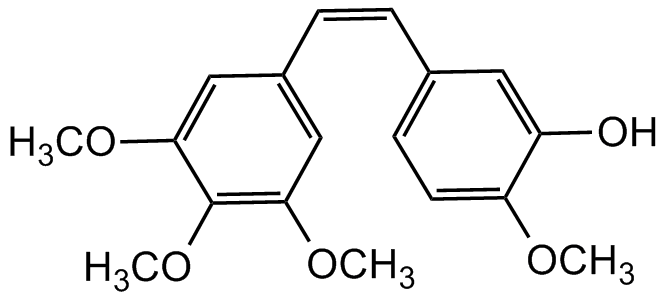
Combretastatin A4
| Code | Size | Price |
|---|
| CDX-C0706-M010 | 10 mg | £114.00 |
Quantity:
| CDX-C0706-M050 | 50 mg | £456.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
1-(3,4,5-Trimethoxyphenyl)-2-(3'-hydroxy-4'-methoxyphenyl) ethane 3,4,5-trimethoxy-3'-hydroxy-4'-methoxystilbene; CA4; CRC 87-09; NSC 817373
Appearance:
Off-white powder.
CAS:
117048-59-6
Class:
6.1
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS05,GHS06
Handling Advice:
Protect from light and moisture.
Hazards:
H301 + H311 + H331-H318
InChi:
InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5-
InChiKey:
HVXBOLULGPECHP-WAYWQWQTSA-N
Long Description:
Chemical. CAS: 117048-59-6. Formula: C18H20O5. MW: 316.35. Combretastatin A4 is a natural stilbenoid phenol originally isolated from an African shrub, Combretum caffrum. It is a potent inhibitor of tubulin polymerization and displays strong inhibitory activity on tumor cell growth. It inhibits tubulin polymerization at the colchicine-binding site of beta-tubulin. CA4 was shown to impede tumor growth in several cell lines including IMR32 (neuroblastoma), Hs746T (gastric carcinoma), CFPAC-1 (pancreatic carcinoma) and MCF-7 (breast cancer). It has antitumor activity by inhibiting AKT function in human gastric cells. The inhibited AKT activation causes decreased cell proliferation, cell cycle arrest and reduced in vitro migration/invasiveness and in vivo metastatic ability. Combretastatin A-4 is the active component of combretastatin A-4 phosphate, a prodrug designed to damage the vasculature (blood vessels) of cancer tumors causing central necrosis. Combretastatin A-4 is therefore a vascular disrupting agent (VDA) that targets tumor vasculature to inhibit angiogenesis.
MDL:
MFCD03453309
Molecular Formula:
C18H20O5
Molecular Weight:
316.35
Package Type:
Vial
PG:
III
Precautions:
P261-P280-P301 + P310-P305 + P351 + P338-P311
Product Description:
Combretastatin A4 is a natural stilbenoid phenol originally isolated from an African shrub, Combretum caffrum. It is a potent inhibitor of tubulin polymerization and displays strong inhibitory activity on tumor cell growth. It inhibits tubulin polymerization at the colchicine-binding site of beta-tubulin. CA4 was shown to impede tumor growth in several cell lines including IMR32 (neuroblastoma), Hs746T (gastric carcinoma), CFPAC-1 (pancreatic carcinoma) and MCF-7 (breast cancer). It has antitumor activity by inhibiting AKT function in human gastric cells. The inhibited AKT activation causes decreased cell proliferation, cell cycle arrest and reduced in vitro migration/invasiveness and in vivo metastatic ability. Combretastatin A-4 is the active component of combretastatin A-4 phosphate, a prodrug designed to damage the vasculature (blood vessels) of cancer tumors causing central necrosis. Combretastatin A-4 is therefore a vascular disrupting agent (VDA) that targets tumor vasculature to inhibit angiogenesis.
Purity:
>98% (HPLC)
Signal word:
Danger
SMILES:
OC1=CC(/C=CC2=CC(OC)=C(OC)C(OC)=C2)=CC=C1OC
Solubility Chemicals:
Soluble in DMSO (10mg/ml), ethanol (5mg/ml) or DMF (10mg/ml). Insoluble in water.
Source / Host:
Plant
Transportation:
Excepted Quantity
UN Nummer:
2811
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) A.T. McGown & B.W. Fox; Anticancer Drug Des. 3, 249 (1989) | (2) R.T. Dorr, et al.; Invest. New Drugs 14, 131 (1996) | (3) J. Griggs, et al.; Int. J. Oncol. 19, 821 (2001) | (4) J. Griggs, et al.; Lancet Oncol. 2, 82 (2011) (Review) | (5) G.C. Tron, et al.; J. Med. Chem. 49, 3033 (2006) (Review) | (6) L.M. Greene, et al.; Biochem. Pharmacol. 84, 612 (2012) | (7) W. Liang, et al.; Med. Sci. Monit. 22, 4911 (2016)