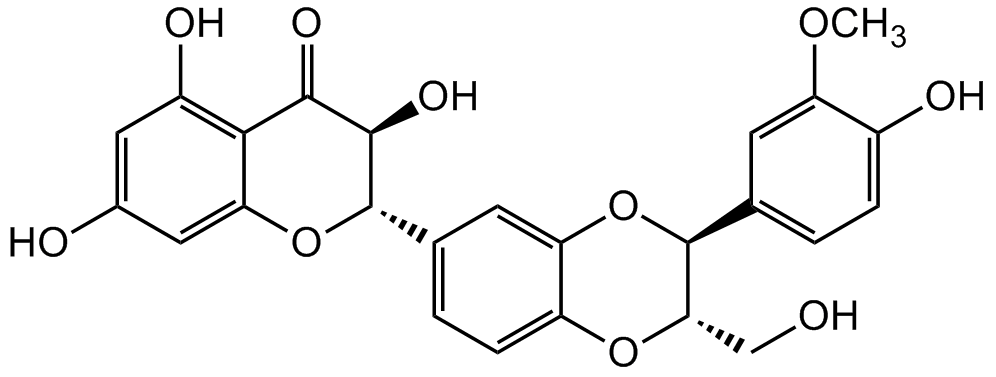
Silibinin
| Code | Size | Price |
|---|
| CDX-S0276-G010 | 10 g | £78.00 |
Quantity:
| CDX-S0276-G100 | 100 g | £547.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-6-(3,5,7-trihydroxy-4-oxobenzopyran-2-yl)benzodioxin; Silybin; NSC 651520; Flavobin; Silymarin I
Appearance:
White to slightly yellow powder.
CAS:
22888-70-6
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07,GHS09
Handling Advice:
Protect from light and moisture.
Hazards:
H302, H410
InChi:
InChI=1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3/t20-,23+,24-,25-/m0/s1
InChiKey:
SEBFKMXJBCUCAI-PQVVKJAFSA-N
Long Description:
Chemical. CAS: 22888-70-6. Formula: C25H22O10. MW: 482.44. Silibinin is a naturally occurring flavanone from Silybum marianum (milk thistle plant) and the main component (active principle) of the Silymarin extract. Silibinin has shown pleiotropic biological properties, including potent antioxidant, anti-inflammatory, anticancer, antidiabetic, hepatoprotective, neuroprotective, antiviral, antimicrobial and immunosuppressive activities. Silibinin demonstrates promising anticancer effects in vitro and in vivo. The molecular mechanisms of silibinin-mediated antiproliferative effects are mainly via receptor tyrosine kinases, androgen receptor, STATs, NF-kappaB, cell cycle arrest and apoptotis/autophagy inducing signaling pathways in various cancer cells. Silibinin was reported to improve glycemic homeostasis by improving the activity of pancreatic beta-cells, increasing insulin sensitivity of liver and muscle cells and decreasing lipid deposition in adipocytes. Next to modulating the NFkappa signaling pathway, Sibilin also inhibits NLRP1/NLRP3 inflammasomes signaling pathways. It modulates many molecular changes caused by xenobiotics and ultraviolet radiation to protect the skin and is used in cosmeceutical preparations.
MDL:
MFCD03225424
Molecular Formula:
C25H22O10
Molecular Weight:
482.44
Package Type:
Vial
Precautions:
P264, P270, P273, P301 + P312, P330, P391, P501
Product Description:
Silibinin is a naturally occurring flavanone from Silybum marianum (milk thistle plant) and the main component (active principle) of the Silymarin extract. Silibinin has shown pleiotropic biological properties, including potent antioxidant, anti-inflammatory, anticancer, antidiabetic, hepatoprotective, neuroprotective, antiviral, antimicrobial and immunosuppressive activities. Silibinin demonstrates promising anticancer effects in vitro and in vivo. The molecular mechanisms of silibinin-mediated antiproliferative effects are mainly via receptor tyrosine kinases, androgen receptor, STATs, NF-kappaB, cell cycle arrest and apoptotis/autophagy inducing signaling pathways in various cancer cells. Silibinin was reported to improve glycemic homeostasis by improving the activity of pancreatic beta-cells, increasing insulin sensitivity of liver and muscle cells and decreasing lipid deposition in adipocytes. Next to modulating the NFkappa signaling pathway, Sibilin also inhibits NLRP1/NLRP3 inflammasomes signaling pathways. It modulates many molecular changes caused by xenobiotics and ultraviolet radiation to protect the skin and is used in cosmeceutical preparations.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
OC1=C2C(O[C@@H](C3=CC=C(O[C@@H](CO)[C@H](C4=CC=C(O)C(OC)=C4)O5)C5=C3)[C@H](O)C2=O)=CC(O)=C1
Solubility Chemicals:
Soluble in DMSO (10mg/ml) or DMF (20mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) C. Dehmlow, et al.; Life Sci. 58, 1591 (1996) | (2) C. Dehmlow, et al.; Hepatology 23, 749 (1996) | (3) J.A. Hannay & D. Yu; Cancer Biol. Ther. 2, 532 (2003) | (4) R.P. Singh & R. Agarwal; Mutat. Res. 555, 21 (2004) (Review) | (5) R.P. Singh, et al.; Oncogene 24, 1188 (2005) | (6) K. Min, et al.; Arch. Pharm. Res. 30, 1265 (2007) | (7) R.P. Singh & R. Agarwal; Clin. Dermatol. 27, 479 (2009) (Review) | (8) L. Li, et al.; Expert Opin. Investig. Drugs 19, 243 (2010) (Review) | (9) F. Yin, et al.; Neurochem. Int. 58, 399 (2011) | (10) G. Marrazzo, et al.; Neurosci. Lett. 504, 252 (2011) | (11) C.O. Souza, et al.; Life Sci. 91, 159 (2012) | (12) J. McClure, et al.; PLoS One 7, e41832 (2012) | (13) D.R. de Oliveira, et al.; Biomed. Res. Int. 2015, 292797 (2015) | (14) N. Polachi, et al.; Eur. J. Med. Chem. 123, 577 (2016) (Review) | (15) J. Bosch-Barrera, et al.; Cancer Treat Rev. 58, 61 (2017) (Review) | (16) L. Tian, et al.; Microb. Pathog. 108, 104 (2017) | (17) R. Faridnia, et al.; Ann. Parasitol. 64, 29 (2018) | (18) C. Chu, et al.; Arch. Pharm. Res. 41, 785 (2018) (Review) | (19) Z. Jahanafrooz, et al.; Life Sci. 213, 236 (2018) (Review) | (20) M.L. Matias, et al.; Molecules 24, E1548 (2019)