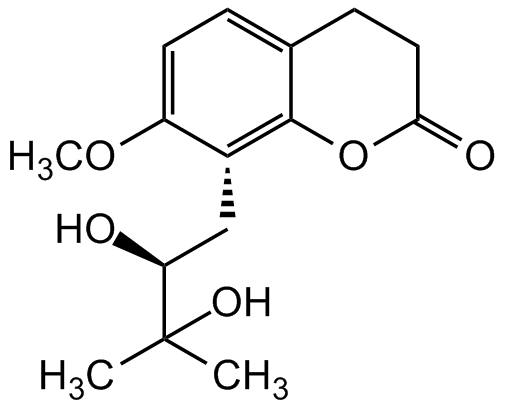
Meranzin hydrate
| Code | Size | Price |
|---|
| CDX-M0513-M001 | 1 mg | £242.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Meracin hydrate
Appearance:
Off-white powder.
CAS:
5875-49-0
Class:
9
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS09
Handling Advice:
Protect from light and moisture.
Hazards:
H361
InChi:
InChI=1S/C15H20O5/c1-15(2,18)12(16)8-10-11(19-3)6-4-9-5-7-13(17)20-14(9)10/h4,6,12,16,18H,5,7-8H2,1-3H3/t12-/m0/s1
InChiKey:
DMVMJLUHWWEOTB-LBPRGKRZSA-N
Long Description:
Chemical. CAS: 5875-49-0. Formula: C15H18O5. MW: 278.3. Meranzin is a bioactive compound from the Traditional Chinese Medicine (TCM) Chaihu-Shugan-San (CSS). Meranzin exhibits anti-inflammatory, analgesic and antibacterial activity. Meranzin regulates the alpha 2-adrenoceptor and involves the AMPA-ERK1/2?BDNF signaling pathway. Meranzin has the potential for the prevention of the comorbidity of atherosclerosis and depression.
MDL:
MFCD18974714
Molecular Formula:
C15H18O5
Molecular Weight:
278.3
Package Type:
Vial
PG:
III
Precautions:
P273-P501
Product Description:
Meranzin is a bioactive compound from the Traditional Chinese Medicine (TCM) Chaihu-Shugan-San (CSS). Meranzin exhibits anti-inflammatory, analgesic and antibacterial activity. Meranzin regulates the alpha 2-adrenoceptor and involves the AMPA-ERK1/2?BDNF signaling pathway. Meranzin has the potential for the prevention of the comorbidity of atherosclerosis and depression.
Purity:
>95% (HPLC)
Signal Word:
Warning
SMILES:
O=C1OC2=C(C[C@@H](C(C)(O)C)O)C(OC)=CC=C2CC1
Solubility Chemicals:
Soluble in DMSO.
Source / Host:
Plant
Transportation:
Non-hazardous
UN Nummer:
3077
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
(1) S. Rosselli, et al.; Planta Med. 73, 116 (2007) | (2) Q.T. Do, et al.; Planta Med. 73, 1235 (2007) | (3) Y. Xie, et al.; Amino Acids 44, 413 (2013) | (4) Y. Xie, et al.; Neuropharmacol. 67, 318 (2013) | (5) L. Li, et al.; Biomed. Pharmacother. 115, 108893 (2019)