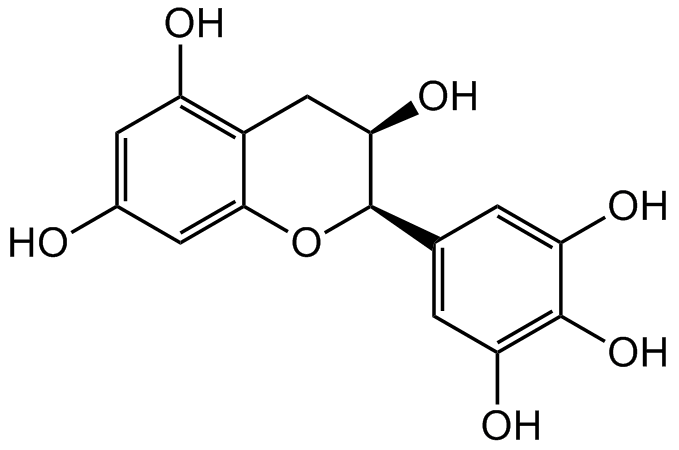
(-)-Epigallocatechin
| Code | Size | Price |
|---|
| CDX-E0182-M010 | 10 mg | £126.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(-)-EGC; epi-Gallocatechin; NSC 674039; (-)-cis-3,3',4',5,5',7-Hexahydroxyflavane; CCRIS 5441
Appearance:
White to beige to pink powder.
CAS:
970-74-1
EClass:
32160000
Form (Short):
solid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1
InChiKey:
XMOCLSLCDHWDHP-IUODEOHRSA-N
Long Description:
Chemical. CAS: 970-74-1. Formula: C15H14O7. MW: 306.27. (-)-Epigallocatechin ((-)-EGC) is a major green tea polyphenol with antioxidant, anti-inflammatory and anticancer activities. It has been shown to scavenge DPPH radicals and to prevent the growth of several different AML cell lines at micromolar concentrations.
MDL:
MFCD00075939
Molecular Formula:
C15H14O7
Molecular Weight:
306.27
Package Type:
Vial
Product Description:
(-)-Epigallocatechin ((-)-EGC) is a major green tea polyphenol with antioxidant, anti-inflammatory and anticancer activities. It has been shown to scavenge DPPH radicals and to prevent the growth of several different AML cell lines at micromolar concentrations.
Purity:
>98% (HPLC)
SMILES:
O[C@H]1[C@@H](C2=CC(O)=C(O)C(O)=C2)OC3=CC(O)=CC(O)=C3C1
Solubility Chemicals:
Soluble in PBS (1mg/ml), ethanol (5mg/ml), DMSO (15mg/ml) or DMF (20mg/ml).
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) J.Z. Xu, et al.; Br, J, Nutr. 91, 873 (2004) | (2) Y. Kushima, et al.; Biol. Pharm. Bull. 32, 899 (2009) | (3) Y. Kadome & S. Fujisawa; Molecules 16, 10457 (2011) | (4) B.T.K. Ly, et al.; PLoS One 8, 1 (2013)