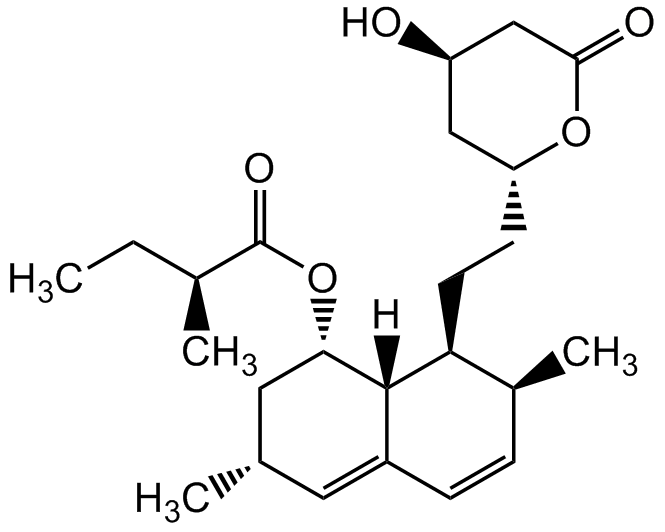
Lovastatin
| Code | Size | Price |
|---|
| CDX-L0281-M025 | 25 mg | £89.00 |
Quantity:
| CDX-L0281-M100 | 100 mg | £280.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Plant
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
Short Term: +20°C, Long Term: +4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Mevilonin; MK-803; 6-alpha-Methylcompactin; BRN 3631989
Appearance:
White to off-white crystalline powder.
CAS:
75330-75-5
EClass:
32160000
Form (Short):
liquid
GHS Symbol:
GHS07
Handling Advice:
Protect from light and moisture.
Hazards:
H302
InChi:
InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
InChiKey:
PCZOHLXUXFIOCF-BXMDZJJMSA-N
Long Description:
Chemical. CAS: 75330-75-5. Formula: C24H36O5. MW: 404.54. Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate. Lovastatin is a prodrug, which is hydrolyzed in vivo to the active beta-hydroxy acid open ring form. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA. Lovastatin is an effective anti-hypercholesterolemic agent widely used as a lipid-lowering drug. In addition to lowering blood lipid levels, Lovastatin also has been shown to have anticancer, neuroprotective, anti-inflammatory, antiviral and antibacterial properties.
MDL:
MFCD00072164
Molecular Formula:
C24H36O5
Molecular Weight:
404.54
Package Type:
Vial
Product Description:
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme that catalyzes the conversion of HMG-CoA to mevalonate. Lovastatin is a prodrug, which is hydrolyzed in vivo to the active beta-hydroxy acid open ring form. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a reversible competitive inhibitor for HMG-CoA. Lovastatin is an effective anti-hypercholesterolemic agent widely used as a lipid-lowering drug. In addition to lowering blood lipid levels, Lovastatin also has been shown to have anticancer, neuroprotective, anti-inflammatory, antiviral and antibacterial properties.
Purity:
>98% (HPLC)
Signal word:
Warning
SMILES:
C[C@H]1C=CC2=C[C@H](C)C[C@H](OC([C@@H](C)CC)=O)[C@]2([H])[C@H]1CC[C@H]3OC(C[C@H](O)C3)=O
Solubility Chemicals:
Soluble in DMSO (20mg/ml), DMF (15mg/ml) or ethanol (20mg/ml). Insoluble in water.
Source / Host:
Plant
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) A.W. Alberts, et al.; PNAS 77, 3957 (1980) | (2) M. Jakobisiak, et al.; PNAS 88, 3628 (1991) | (3) P. Negre-Aminou, et al.; Biochim. Biophys. Acta 1345, 259 (1997) | (4) B. Kwak, et al.; Nat. Med. 6, 1399 (2000) | (5) C. Wojcik, et al.; Int. J. Biochem. Cell Biol. 32, 957 (2000) | (6) L.J. Raggatt & N.C. Partridge; Drugs 62, 2185 (2002) (Review) | (7) W.W. Wong, et al.; Leukemia 16, 508 (2002) | (8) K.K. Chan, et al.; Clin. Cancer Res. 9,10 (2003) | (9) L.M. Blanco-Colio, et al.; Kidney Int. 63, 12 (2003) | (10) J.A. Tobert; Nat. Rev. Drug Discov. 2, 517 (2003) (Review) | (11) V. Chopra, et al.; Cardiovasc. Drugs Ther. 21, 161 (2007) | (12) Z. Xiong, et al.; Food Chem. Toxicol. 131, 110585 (2019) (Review)